��Ŀ����
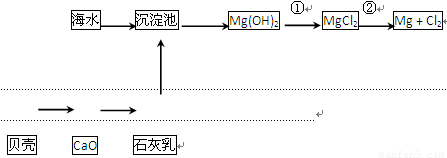
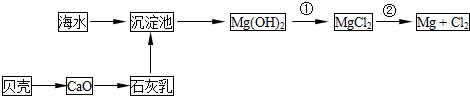
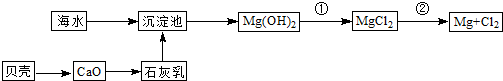
�Ӻ�ˮ�п��Ի�õ�ˮ��ʳ�Σ�������ȡþ��������ʣ����к�ˮ�����ij��÷�����Ҫ�� ����һ�֣���
�Ӻ�ˮ����ȡ���þ����������ͼ��ʾ��
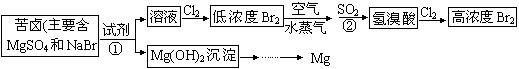
��1����ȡBr2ʱ��һ��ͨ��Cl2������Ӧ�����ӷ���ʽ�� ����Ӧ�Т���SO2���ֳ�___ �����������ԭ�����ԣ�
�ڶ���ͨ��Cl2��Ҫ��ȡBr2��Ҫ���еIJ���Ϊ ]
��2��Ϊ��ʵ�ֶ�þ���ӵĸ��������м���������Լ��� _____ ���ѧʽ����
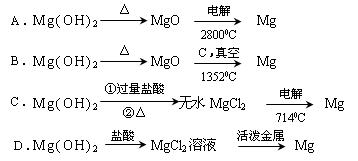
�Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ��������������˵�ұ���ķ����� ������ĸ����
A��Mg��OH��2![]() MgO
MgO![]() Mg
Mg
B��Mg��OH��2![]() MgO
MgO![]() Mg
Mg
C��Mg��OH��2![]() ��ˮMgCl2
��ˮMgCl2![]() Mg
Mg
D��Mg��OH��2 ![]() MgCl2��Һ
MgCl2��Һ![]() Mg
Mg
�����������������ӽ��������δ�һ�֣�
��1��Cl2 + 2Br��= 2Cl��+ Br2 ��2�֣�����ԭ��1�֣�����ȡ����Һ��2�֣�
��2��Ca��OH��2����NaOH�����������𰸾����֣���1�֣���C ��1�֣�
����:
��

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д�
�����Ŀ
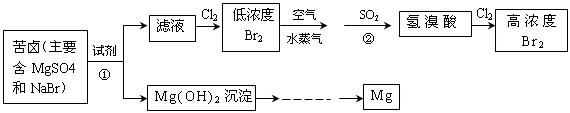
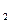 ʱ��һ��ͨ��Cl
ʱ��һ��ͨ��Cl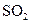 ���� �����������ԭ�����ԣ�
���� �����������ԭ�����ԣ�