��Ŀ����
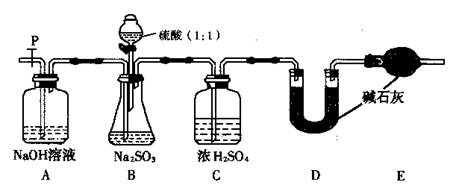
(14��)���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�����ⶨ���������Լ��������ij̶ȣ��������ͼʵ�飬��ش���������⣺
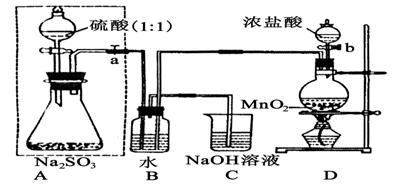
(1)Dװ���з�Ӧ�Ļ�ѧ����ʽΪ ��
Bװ���з�Ӧ�����ӷ���Ϊ ��
(2)����ag Na2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м���������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����ð�ɫ����bg��ԭ��Ʒ��Na2SO3��������Ϊ��Ϊ�� ��
(3)Ϊ��֤ʵ��ⶨ��ȷ�ԣ�A�е�����ʲôʱ��μ�
Cװ���е������� _________________��
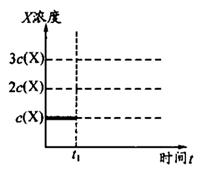
(4)���������Լ���������ˮ����ϡ���ᡢ��ϡ���ᡢ��BaCl2��Һ����Ba(NO3)2��Һ�������ѡ������Լ������һ�ֲ�ͬ��ʵ�鷽�����ⶨ��������ˮ�������Ʊ������ij̶ȣ���ʹ���Լ���˳��Ϊ�� ��(���Լ����)
(1)MnO2��4HCl(Ũ) MnCl2��Cl2����2H2O ��Cl2+SO2+2H2O=4H��+2Cl��+SO42-
MnCl2��Cl2����2H2O ��Cl2+SO2+2H2O=4H��+2Cl��+SO42-
�� (2) 
(3)��B��ˮ���Ϸ������˻���ɫ������ ����β������ֹ��Ⱦ����
(4)�٢ڢ�
��������
�����������1��װ��D����������װ�ã������ķ�ӦΪMnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O��װ��B����������SO2�ķ�Ӧ������ʽΪCl2+SO2+2H2O=4H��+2Cl��+SO42-��
MnCl2+Cl2��+2H2O��װ��B����������SO2�ķ�Ӧ������ʽΪCl2+SO2+2H2O=4H��+2Cl��+SO42-��
��2���õ���bg������BaSO4������Na2SO3��SO2��BaSO4����֪Na2SO3Ϊ =
=  ������Na2SO3��������Ϊ��Ϊ
������Na2SO3��������Ϊ��Ϊ ��
��
��3����֤ʵ���ȷ�Ծ��Dz���SO2��ʧ������������Ӧ����B��ˮ���Ϸ������˻���ɫ�������ʱ��μӡ�C�е�NaOH������β������ֹ��Ⱦ������
��4������һ����Ʒ����ǽ�Na2SO3����ˮ��Ȼ�������м���ϡ���������ٲ�������Ϊֹ���ڼ�BaCl2��Һ��Ȼ�����ɵij�������ϴ�Ӹ�����������������Na2SO4�������������̶����Na2SO3����������������ʹ���Լ���˳���Ǣ٢ڢܡ�
���㣺SO2��Cl2������
�����������ۺ���ǿ����Ҫ����ѧ������ʵ�����ʵ���������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д�
 Ԫ��,
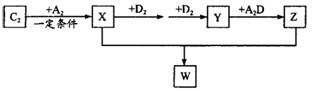
Ԫ��, ��A2Ϊ���³�ѹ���ܶ���С�����壬B�ĵ����ж���ͬ�������壬����һ��Ϊ�����Һ�ɫ���塣C�������������ǵ��Ӳ�����2. 5������E�ĵ��������IJ;߲��˳��ڴ�����ԡ����Ի���ζʳ�
��A2Ϊ���³�ѹ���ܶ���С�����壬B�ĵ����ж���ͬ�������壬����һ��Ϊ�����Һ�ɫ���塣C�������������ǵ��Ӳ�����2. 5������E�ĵ��������IJ;߲��˳��ڴ�����ԡ����Ի���ζʳ�
 Ԫ��,
Ԫ��, ��A2Ϊ���³�ѹ���ܶ���С�����壬B�ĵ����ж���ͬ�������壬����һ��Ϊ�����Һ�ɫ���塣C�������������ǵ��Ӳ�����2. 5������E�ĵ��������IJ;߲��˳��ڴ�����ԡ����Ի���ζʳ�
��A2Ϊ���³�ѹ���ܶ���С�����壬B�ĵ����ж���ͬ�������壬����һ��Ϊ�����Һ�ɫ���塣C�������������ǵ��Ӳ�����2. 5������E�ĵ��������IJ;߲��˳��ڴ�����ԡ����Ի���ζʳ�