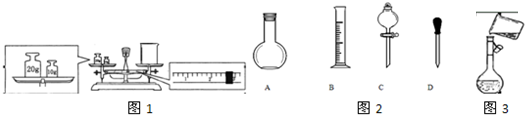
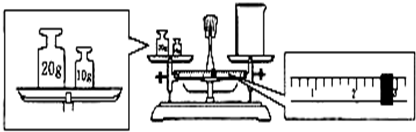
��Ŀ����
��12�֣� ijͬѧ��ʵ�������������ʵ���Ũ�Ⱦ�Ϊ1��0 mol/L��NaOH��Һ��ϡH2SO4��450mL���ṩ���Լ��ǣ�NaOH�����98%��ŨH2SO4���ܶ�Ϊ1��84 g/cm3��������ˮ��
��1������������Һʱ����Ҫ�IJ���������____________________________________ ��
��2��Ӧ��������ƽ����NaOH ___________g��Ӧ����Ͳ��ȡŨH2SO4________mL��
��3������ʱ����Ҫ�������ƿ�Ƿ�©ˮ���䷽����
 ��
��
��4��Ũ��������ˮ����ȷ����������_____ ______ _____��
_____��
��5��������������Һʵ���У����в���������ƫ�͵���_________________
| A����ѧ������ȡŨ����ʱ�����ӿ̶��� |
| B����������NaOHʱ�����������Ʒ��λ�õߵ���û��ʹ�����룩 |
C���ܽ�H2SO4����ʱû����ȴ�����¾�������ɺ�������Ʋ� ���� ���� |
D�����ձ����ܽ� ����ʱ������������Һ ����ʱ������������Һ |
F������Ͳϴ��2��3�Σ���ȫ��ת��������ƿ��
G������ƿ��ԭ��������������ˮ
H����ͷ�ιܼ�ˮ����ʱ���ӿ̶�

����

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�| A������������ƽ�ϳ�ȡԼ1.7��Na2CO3���� | B��ϴ���ձ������Һ�ò���������������ƿ�� | C��ϴ��2-3�κ�������ˮ�ز�����ע������ƿ���̶��� | D��ijͬѧ�������ӵķ�ʽ���ݣ����������ҺŨ��ƫ�� |
��19�֣�����������̵���Ҫ������ͳ��õ���������������ʵ������ģ�ҵ�������̿��Ʊ�������ص�����ͼ��
��1�������������Ϊ �������������Ϊ ��
��2����Ӧ�ڵĻ�ѧ����ʽΪ ����ҵ��������ԭ������KMnO4�������ʽϵͣ��Ϻõ��Ʊ������ǵ�ⷨ����Pt��������Fe��������K2MnO4Ϊ���Һ�������ĵ缫��ӦʽΪ ��
��3��KMnO4��һ�ֽ��ȶ��Ļ�������չ��KMnO4��Һ�ķֽ��д����ã�����MnO2��KOH��O2����MnO2Ҳ�Ǹ÷ֽⷴӦ��һ�ִ������������һ��ʵ�鷽������֤MnO2�Ը÷ֽⷴӦ���д��ԣ� ��
��4��KMnO4�����Խ����е�ǿ�����Թ㷺Ӧ���ڷ�����ѧ�С�
���磺2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O��ijͬѧ��KMnO4�ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȡ�����ȷ��ȡ6.3 gNa2SO3������Ʒ�����500 mL��Һ��ȡ25.00 mL������Һ������ƿ�У���0.01000 mol/L ������KMnO4��Һ���еζ����ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 mL | 0.02 | 24.01 |
| 2 | 25.00 mL | 0.70 | 24.71 |
| 3 | 25.00 mL | 0.20 | 24.20 |
������500 mLNa2SO3��Һʱ�������õ���ʵ�������У��ձ�������������ͷ�ιܡ�ҩ�� �� ��
���жϵζ��յ�������� ��
�����в����ᵼ�²ⶨ���ƫ�ߵ���
A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ���
B���ζ�ǰ��ƿδ����
C���ζ�ǰ�ζ��ܼ��첿��������
D���۲����ʱ���ζ�ǰ���ӣ��ζ�����
��������ʵ�����ݣ�����Na2SO3�Ĵ���Ϊ ��