��Ŀ����
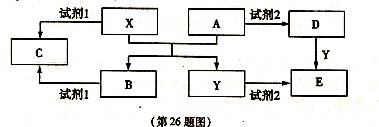
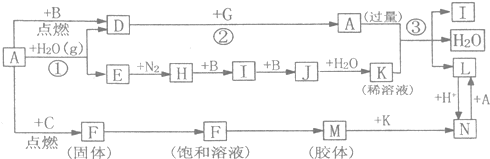
��ͼ����ѧ��ѧ�������ʵ�ת����ϵ��ijЩ��Ӧ���������ֲ�������ȥ����A��GΪ�ճ������еij���������B��C��E��I��JΪ���壬����CΪ����ɫ���壬MΪ���ɫ���壮
��1��д��AԪ�������ڱ��е�λ��______��ԭ�ӽṹʾ��ͼ��______��
��2��д����Ӧ�ڵĻ�ѧ����ʽ______��ʵ�����������÷�Ӧ�IJ��������ǣ�______��
��3����Ӧ�ٻ�ѧ����ʽ______����Ӧ�����ӷ���ʽ______��
��4��ʵ�����Ʊ�����H�Ļ�ѧ����ʽ��______����������H��ѡ��______�������ƣ����������
��5���ֽ�һ�Թ�����J������ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����______���ѧʽ������ʱ�Թ�����Һ��Ũ��Ϊ��______mol/L�������������״�����㣩��������λ��Ч���֣���
�⣺A��GΪ�ճ������еij���������B��C��E��I��JΪ�������CΪ����ɫ�����ж�ΪCl2��MΪ���ɫ�����ж�ΪFe��OH��3���ƶ�FΪFeCl3��AΪFe�����ݷ�Ӧ�����õ�DΪFe3O4��BΪO2��EΪH2��HΪNH3��IΪNO��JΪNO2��KΪHNO3����������ϡ���ᷴӦ��������������һ��������ˮ������ת����ϵ�еķ�Ӧ������ϵ�ж�NΪFe��NO3��3��LΪFe��NO3��2��
��1��AԪ����Fe�������ڱ��е�λ��Ϊ���������� �ڢ����壬ԭ��������26��ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ���������� �ڢ�����
���ʴ�Ϊ���������� �ڢ����� 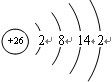
��2����Ӧ��Ϊ����������������Ӧ������������������Ӧ�Ļ�ѧ����ʽ��8Al+3Fe3O4 4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ����
�ʴ�Ϊ��8Al+3Fe3O4 4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ����
��3����Ӧ��������ˮ�������·�Ӧ������������������������ѧ����ʽΪ��3Fe+4H2O Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
�ʴ�Ϊ��3Fe+4H2O Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
��4��ʵ�����Ʊ�����H��NH3���������Ȼ�粒�����������Ƽ��ȷ�Ӧ����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ��
CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl CaCl2+2NH3��+2H2O����ʯ�ң�
CaCl2+2NH3��+2H2O����ʯ�ң�
��5���ֽ�һ�Թ�����J��NO2��������ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����������������Ӧ��4NO2+O2+2H2O=4HNO3����ҺŨ�����ݻ�ѧ����ʽ���м���õ���4NO2+O2+2H2O=4HNO3����ԭ�Թ��ж��������������ΪVL�������ʵ���Ϊ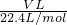 ��������Һ���������ʵ���Ϊ
��������Һ���������ʵ���Ϊ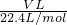 ����ҺŨ��=
����ҺŨ��=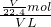 =0.045mol/L���ʴ�Ϊ��0.045mol/L��
=0.045mol/L���ʴ�Ϊ��0.045mol/L��
����������CΪ����ɫ�����ж�ΪCl2��MΪ���ɫ�����ж�ΪFe��OH��3���ƶ�FΪFeCl3��AΪFe�����ݷ�Ӧ�����õ�DΪFe3O4��BΪO2��EΪH2��HΪNH3��IΪNO��JΪNO2��KΪHNO3����������ϡ���ᷴӦ��������������һ��������ˮ������ת����ϵ�еķ�Ӧ������ϵ�ж�NΪFe��NO3��3��LΪFe��NO3��2���ۺ�ת����ϵ�����Ƶ�����Ҫ�������жϳ������ʽ��з����ش����⣻
���������⿼��������ת����ϵ���жϣ��������ʵ�Ӧ�ã���Ӧ�����Ӧ�ã���Ҫ���������仯�������ʣ������仯�������ʵ��ۺ�Ӧ�ã�����ʵ�����Ʊ��������������������ͨ��ˮ�е�Ũ�ȼ��㣬�ۺ��Խ�ǿ��
��1��AԪ����Fe�������ڱ��е�λ��Ϊ���������� �ڢ����壬ԭ��������26��ԭ�ӽṹʾ��ͼΪ��
 ���ʴ�Ϊ���������� �ڢ�����
���ʴ�Ϊ���������� �ڢ����� 
��2����Ӧ��Ϊ����������������Ӧ������������������Ӧ�Ļ�ѧ����ʽ��8Al+3Fe3O4
 4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe��ʵ�����������÷�Ӧ�IJ����������������ȼ������һ������أ���һ��þ������ȼþ�����ʴ�Ϊ��8Al+3Fe3O4
 4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ����
4Al2O3+9Fe���������ȼ������һ������أ���һ��þ������ȼþ������3����Ӧ��������ˮ�������·�Ӧ������������������������ѧ����ʽΪ��3Fe+4H2O
 Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2����Ӧ�����ӷ���ʽ��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O���ʴ�Ϊ��3Fe+4H2O
 Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O��
Fe3O4+4H2��3Fe+8H++2NO3-=3Fe2++2NO��+4H2O����4��ʵ�����Ʊ�����H��NH3���������Ȼ�粒�����������Ƽ��ȷ�Ӧ����Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ��
CaCl2+2NH3��+2H2O�������Ǽ�����������ü�ʯ�Ҹ���ʴ�Ϊ��Ca��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O����ʯ�ң�
CaCl2+2NH3��+2H2O����ʯ�ң���5���ֽ�һ�Թ�����J��NO2��������ˮ����һ��ʱ���ˮ������������������ʹˮ���������Թܣ�Ӧ���Թ���ͨ��һ����������������Ӧ��4NO2+O2+2H2O=4HNO3����ҺŨ�����ݻ�ѧ����ʽ���м���õ���4NO2+O2+2H2O=4HNO3����ԭ�Թ��ж��������������ΪVL�������ʵ���Ϊ
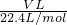 ��������Һ���������ʵ���Ϊ
��������Һ���������ʵ���Ϊ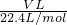 ����ҺŨ��=
����ҺŨ��=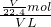 =0.045mol/L���ʴ�Ϊ��0.045mol/L��
=0.045mol/L���ʴ�Ϊ��0.045mol/L������������CΪ����ɫ�����ж�ΪCl2��MΪ���ɫ�����ж�ΪFe��OH��3���ƶ�FΪFeCl3��AΪFe�����ݷ�Ӧ�����õ�DΪFe3O4��BΪO2��EΪH2��HΪNH3��IΪNO��JΪNO2��KΪHNO3����������ϡ���ᷴӦ��������������һ��������ˮ������ת����ϵ�еķ�Ӧ������ϵ�ж�NΪFe��NO3��3��LΪFe��NO3��2���ۺ�ת����ϵ�����Ƶ�����Ҫ�������жϳ������ʽ��з����ش����⣻
���������⿼��������ת����ϵ���жϣ��������ʵ�Ӧ�ã���Ӧ�����Ӧ�ã���Ҫ���������仯�������ʣ������仯�������ʵ��ۺ�Ӧ�ã�����ʵ�����Ʊ��������������������ͨ��ˮ�е�Ũ�ȼ��㣬�ۺ��Խ�ǿ��

��ϰ��ϵ�д�
�����Ŀ

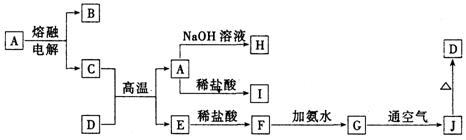
 ��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����
��ϵ����ͼ��ʾ�����ַ�Ӧ�����������ȥ����