��Ŀ����
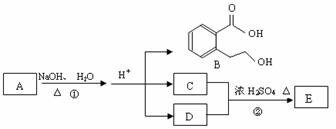
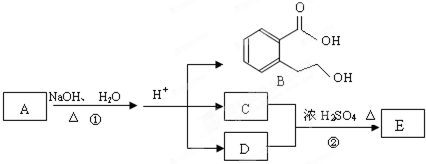
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���úͣĵ���Է���������ȣ�
��EΪ��֧���Ļ����
������ͼ�ش����⣺
����1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪ������������������ ���� C�����еĹ����������� ______________��������B���������ķ�Ӧ�������������������� ������ĸ��ţ���
a���ӳɷ�Ӧ ������b��ȡ����Ӧ ��������c����ȥ��Ӧ
d��������Ӧ ������e��ˮ�ⷴӦ ��������f�� �û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��_________________���������������������������� _��
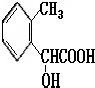
��3��A�Ľṹ��ʽ�� __________________������������������ ��
��4��ͬʱ������������������B��ͬ���칹�����Ŀ���������� ����
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3 ��Һ������ɫ��Ӧ��
д��������������ͬ���칹��Ľṹ��ʽ������������ �������� ������������ ��
��(1) C5H10O2 (2��) ��2���Ȼ� (2��) e (2��)
��2��CH3COOH + CH3CH2CH2OH![]() CH3COOCH2CH2CH3 + H2O (2��)
CH3COOCH2CH2CH3 + H2O (2��)
��3�� 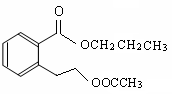 ��
�� (2��)
(2��)
��4��4

д������֮�����ɣ�ÿ��2�֣���6�֣�
|


 ����1��
����1��



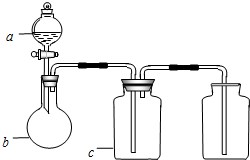 ��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������
��ͼ��a��b��c��ʾ��Ӧ�����м�����Լ���������ͼװ����ȡ���������ռ��������ǣ�������


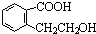
 CH2-CH2
CH2-CH2 n��CH2=CH2+H2O
n��CH2=CH2+H2O