��Ŀ����
ij��Һ�п��ܺ�������6�������еļ��֣�NH4+��A13+��Mg2+��CO32�D��Clһ��SO42�D��Ϊȷ����Һ��ɣ���ȡ100 mL�ֳ����ȷ���Һ��������ʵ�飺
��1�����һ����Һ�м��� AgNO3��Һ�г���������
��2����ڶ�����Һ�м�������NaOH��Һ��ַ�Ӧ�����յõ�����0.58 g(�����ˡ�ϴ�ӡ������ͬ)��ͬʱ�ռ�������0.03 mol(������ȫ������Һ���ݳ�)��
��3�����������Һ�м�������BaCl2��Һ(�����ữ)��ַ�Ӧ�õ�����6.99 g��
�ɴ˿�֪�����й���ԭ��Һ��ɵ���ȷ������
| A��һ������Clһ |
| B��һ������A13+��NH4+ |
| C��һ��������Mg2+�����ܴ���A13+ |
| D����Һ��SO42�D��Ũ����0. 3 mol/L |
B
����������������� AgNO3��Һ�г�����������ȷ����Һ���Ƿ�һ������Clһ��Ϊ̼����Ҳ�ǰ�ɫ������A����������þ�������������ƣ����Լ���������������Ʋ�������˵����Һ��һ������Mg2+����Һ�м�������BaCl2��Һ(�����ữ)��ַ�Ӧ�õ����ᱵ����6.99 g�����ʵ���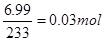 ������Ԫ���غ���������ӵ����ʵ���Ϊ
������Ԫ���غ���������ӵ����ʵ���Ϊ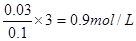 ��D�������Դ�ѡB��
��D�������Դ�ѡB��
���㣺�й����ӹ��淽��ļ����жϡ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����������ӷ���ʽ����д��ȷ����
A������������������ |
B����ˮ��ͨ������������ |
C��̼��������Һ�м������ |
D��������Һ�м������ ��Һ�� ��Һ�� |
������Һ���ܴ�����������ҺΪ��ɫ������������ �� ��
| A��K+��MnO4����Na+��Cl�� | B��K+��Na+��NO3����CO32�� |
| C��Na+��H+��NO3����SO42�� | D��Fe3+��Na+��Cl����SO42�� |
���и���Һ�е��������ܴ���������ǣ� �� ��
| A��pH=0����Һ�У�K+��Fe2+��C1����NO3�� |
| B��ǿ������Һ�У�Na+��K+��A1O2����CO32�� |
| C��c��H+��=c��OH-������Һ�У�Na+��Ba2+��C1����SO42- |
| D��ʹ��ɫʯ����Һ������Һ�У�Na+��K+��SO32����HCO3�� |
���и��������У��ڼ�����Һ���ܹ��棬�Ҽ�����������л��������ͳ�������
| A��K+��Na+��AlO2����CO32�� | B��Na+��Cl����SiO32����K+ |
| C��Na+��NO3����AlO2����K+ | D��Na+��Cl����HCO3����Ba2+ |
����ɫ����Һ�У����и����е�����һ���ܴ����������
| A��K+��Fe2+��NO3����H+ | B��Na+��Ca2+��Cl����NO3�� |
| C��Na+��H+��Cl����HCO3�� | D��Na+��Cu2+��OH����SO42�� |
�����£����и���������ָ����Һ��һ���ܴ����������
| A����ɫ������Һ�У�Cu2+��Fe3+��SO42-��OH- |
| B��ʹ��̪���ɫ����Һ�У�Na����K����CO32-��NO3- |
| C�����д���Ba��NO3��2����Һ�У�Mg2+��NH4+��SO42-��Cl- |
| D��0. 1 mol��L-1AlCl3��Һ�У� K����NH4����HCO3-��SO42- |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��NaHCO3��Һ�������Һ��Ӧ��HCO3�� + H+="==" CO2��+H2O |
| B���Ȼ�����Һ�������ˮ��Ӧ��Al3+ + 4OH��="==" AlO2��+ 2H2O |
| C��Na2O2����ˮ����O2��Na2O2+H2O ="==" 2Na��+2OH��+O2�� |
D��NaOH��Һ���Ȼ����Һ�ڼ����·�Ӧ��NH4��+OH�� NH3��+H2O NH3��+H2O |
�������ӷ���ʽ��д��ȷ����
| A������ʯ��ˮ������С�մ���Һ��ϣ�Ca2+ +2OH- +2HCO3- = CaCO3��+ CO32- + 2H2O |
| B���Ȼ�����Һ�м�������İ�ˮ��Al3+ + 4NH3��H2O = Al(OH)4-+ 4NH4+ |
| C��Ư����Һ��ͨ������SO2���壺Ca2+ +2ClO- +SO42- +H2O =CaSO3��+2HClO |
| D����NH4HSO4ϡ��Һ����μ���Ba(OH)2ϡ��Һ��SO42-�պó�����ȫ�� Ba2+ + 2OH- + NH4+ + H+ + SO4 2- = BaSO4��+ NH3��H2O + H2O |