��Ŀ����
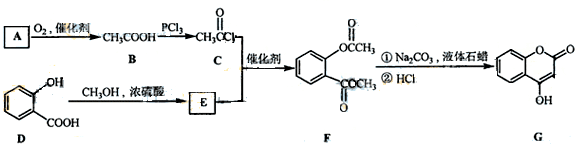
�л���A��B��C��D��E��F��G�����ϵ����ͼ��ʾ��
��1������A��±Ԫ�ص�ʵ�鷽���� ��
��2��B�Ľṹ��ʽΪ ���ٵĻ�ѧ��Ӧ������ ��
��3��G��ʵ�����п�ͨ��������Ӧ������F�����е�һ����Ӧ�������� ����Ӧ�õ��IJ�������У���д�ṹ��ʽ�� ��
��4��F��һ�ֶ�Ԫ�ᣬ����һ�������¿���G��Ӧ���ɸ߷��ӻ�����ø߷��ӵĽṹ��ʽΪ ��
д����Ӧ�ܵĻ�ѧ��Ӧ����ʽ ��
��1��ȡ����A���Թܣ���������������Һ����һ��ʱ�䣬��ȴ������ϡ��������Һ��������������������Һ������е���ɫ�������֣���A�к���Ԫ�ء���2�֣�
��2��H2C=CH-COOH��1�֣��� ��ȥ��������Ӧ��1�֣�
��3��Cu��Ag�����������ȣ�1�֣��� HO-CH2-CH2-CHO��OHC-CH2-CHO��2�֣�
��4��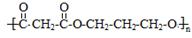 ��1�֣�
��1�֣�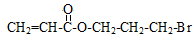 +2NaOH
+2NaOH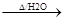 CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣�
CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣�
���������������1��±������±�����ӵļ��鷽���ǣ�ȡ����A���Թܣ���������������Һ����һ��ʱ�䣬��ȴ�����ϡ��������Һ�����ԣ��μ���������Һ������е���ɫ�������֣���A�к���Ԫ�ء�����A��������������������������ʣ���֪�Ƿ�����ˮ�ⷴӦ��D��B���ữ��˵��B���ᣬ��C�Ǵ�����̼ԭ������̼�ĹǼ���ȫ��ͬ������ת����ϵ��֪BΪH2C=CH-COOH��F��һ�ֶ�Ԫ�ᣬͨ��ת����ϵ�ɵó�CΪBrH2C-CH2-CH2OH��EΪBrH2C-CH2-CHO��DΪH2C=CH-COONa��GΪHOH2C-CH2-CH2OH,FΪHOOC-CH2-COOH��
��2��B�Ľṹ��ʽΪH2C=CH-COOH���ٵĻ�ѧ��Ӧ��������ȥ��������Ӧ��G��ʵ�����п�ͨ��������Ӧ������F�����е�һ����Ӧ��������Cu��Ag�����������ȣ���Ӧ�õ��IJ����в���������ȫ���������ʲ��������HO-CH2-CH2-CHO��OHC-CH2-CHO��
��4��F��һ�ֶ�Ԫ�ᣬ����һ�������¿���G��Ӧ���ɸ߷��ӻ�����൱�ڷ�����������Ӧ�����˸߾��д����Ӧ��Ϊ����±������ˮ�ⷴӦ�������������ŷ����˱仯�����㣺���⿼�����л���ͼ�ƶϣ���һ���ۺ��Ե�ǰ���ƶϣ��ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���A�����������ʵ���1:1�����ӳɷ�Ӧʱ���Եõ����ֲ���������������ֲ���Ľṹ��ʽ��
| ���� | �� | �� |
| �ṹ��ʽ |  | 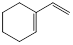 |
��1�����������ӳɲ����⣬��д�������ӳɲ���Ľṹ��ʽ��___________�������������칹����
��2������A�������ڷ�������ͬ���칹����Լ���________________��д��ѧʽ����
��3����֪����˫�������Ը��ڻ���˫����д������ �� �����ۺϷ�Ӧ���ò���Ľṹ��ʽ��
_________________��
��4��˫ϩ�ϳɷ�Ӧ����������ʾ��
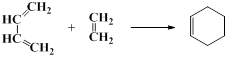
д������ �� ����ϩ����˫ϩ�ϳɷ�Ӧ����Ľṹ��ʽ��______________��
��5���Բ��� �� Ϊԭ��ͨ��������Ӧ����ת��Ϊ2-��-3-�һ�-1,4-����������
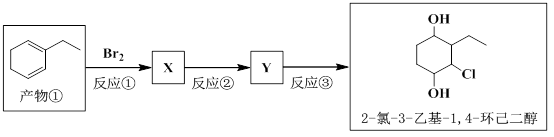
д����Ӧ �� �Ļ�ѧ����ʽ��_____________________��

 ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ����___________________________________________________��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ����___________________________________________________�� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����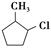 Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
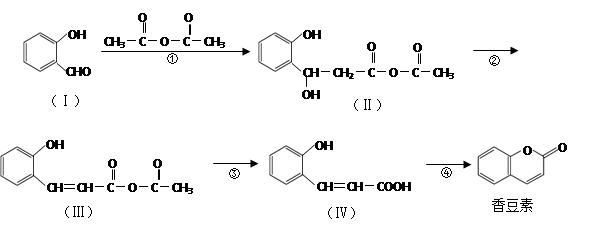
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�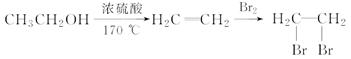
 ��CH3CHO�ܷ������Ʒ�Ӧ�١��ڵ�������Ӧ���������ɵ��л���Ľṹ��ʽΪ ��
��CH3CHO�ܷ������Ʒ�Ӧ�١��ڵ�������Ӧ���������ɵ��л���Ľṹ��ʽΪ ��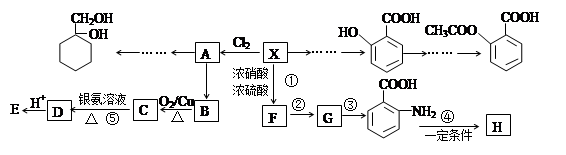
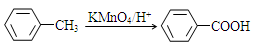
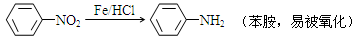
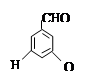 �ж���ͬ���칹�壬д��2�ֺ���1��ȩ����2���ǻ��ұ�����ֻ��2��һ��ȡ����ķ����廯����Ľṹ��ʽ�� ��
�ж���ͬ���칹�壬д��2�ֺ���1��ȩ����2���ǻ��ұ�����ֻ��2��һ��ȡ����ķ����廯����Ľṹ��ʽ�� �� ��·�ߡ�����A�� ���� ��
��·�ߡ�����A�� ���� ��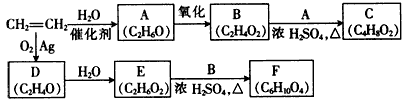
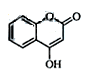 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�