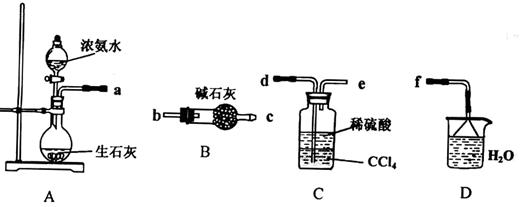
��Ŀ����
(15��)����������ֳ�Ī���Σ���dz��ɫ���塣���ڿ����б�һ���������ȶ����dz��õ�Fe2+�Լ���ijʵ��С�����ù�ҵ����м��ȡĪ���Σ����ⶨ�䴿�ȡ�
��֪:��
��Ī�������Ҵ��ܼ������ܡ�
��Ī���ε���ȡ
�Է�����
��1������2�м��ȷ�ʽ ���ֱ�Ӽ��ȡ��p��ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���� ��
��2������3�а�����ʵ��������� ��
��3����ƷĪ��������� ϴ�ӣ�����ĸ��ţ���
a������ˮ b���Ҵ� c����Һ
��Ϊ�ⶨ���������(NH4)2SO4?FeSO4?6H2O���崿�ȣ�ijѧ��ȡm g�����������Ʒ���Ƴ�500 mL��Һ������������ɣ��ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
(��)����һ��ȡ20.00 mL�����������Һ��0.1000 mol��L��1������KMnO4��Һ�����ν��еζ���
(��)��������ȡ20.00 mL�����������Һ��������ʵ�顣
��1����ʵ���������ȷ��������һ�IJⶨ�������С�ڷ������������ԭ��Ϊ
����֤�Ʋ�ķ���Ϊ�� ��
(��)��������(ͨ��NH4+�ⶨ)ʵ�����ͼ������ʾ��ȡ20.00 mL�����������Һ���и�ʵ�顣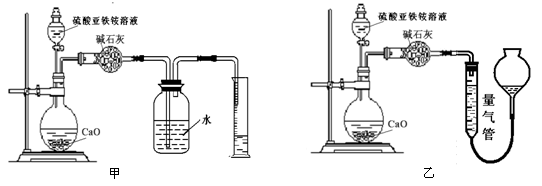
��2����װ�� ����ס����ҡ�����Ϊ�������ж������� ��������������Լ��� ������ĸ��š���ѡ���ҡ�����˿գ���ѡ���ס��˿տɲ����
a��ˮ b������NaHCO3��Һ c��CCl4
�������NH3�����ΪV L(������Ϊ��״����)�������������茶���Ĵ���Ϊ ��
��15�֣�
I����1��ˮԡ����(1�� )
��ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������2�֣�
��2������������Ũ���ᾧ��2�֣�
��3�� b��1�� ��
II����1�� Fe2+�ѱ������������� (2��)
ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+��
�������������� (2��)
��2�����ң�1�֣� ��װ�û���ֵ���(1��) c ��1�֣�
�� (2��)
(2��)
�������������I.��1������2�е��¶ȿ�����70-75�棬����ѡ��ˮԡ���ȣ�Fe2+�ױ����������������¶Ƚ��������������ܽ�ȼ�С�����Ա�������м����ʣ��ʱ�������ȹ��ˣ���ԭ���Ƿ�ֹFe2+�ױ�������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������
��2���������������Һ�����壬�м���Ҫ��������������Ũ���ᾧ��
��3����ΪĪ�������Ҵ��ܼ������ܣ�����ѡ�����Ҵ�ϴ�ӣ���ѡb��
II.��1�������Ը��������Һ�ζ���ԭ�����������ӱ����Ը��������Һ����������������Һ����������������ӵ������Ӷ����㾧��Ĵ��ȣ����Ȼ�����Һ�ζ���ԭ��������������뱵���ӷ�Ӧ�������ᱵ���������ó���������������������ӵ������Ӷ����㾧��Ĵ��ȡ���������Ӳ��ᷢ���仯�����Է���һ�IJⶨ�������С�ڷ������������ԭ��ΪFe2+�ѱ�����������������֤�Ʋ�ķ���������֤��Һ���Ƿ���������ӣ����������ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+�ѱ���������������
��2����װ���ұȽϺ�������Ϊ��װ�û���ֵ��������������������������ѡ����װ�ã�����������ʢ�ŵ��Լ������Ȼ�̼����Ϊ����������ˮ�����������Ȼ�̼���������ݱȽ�ȷ�����Դ�ѡc��
�ڸ�����������淋Ļ�ѧʽ�ó�2NH3��(NH4)2SO4?FeSO4?6H2O������500mL��Һ����������茶��������ΪVL/22.4L/mol/2��392g/mol��25���䴿��ΪVL/22.4L/mol/2��392g/mol��25/mg��100%= ��
��
���㣺���������Ʊ����������жϣ����ӵļ��飬���ȵļ���

 �Ķ��쳵ϵ�д�
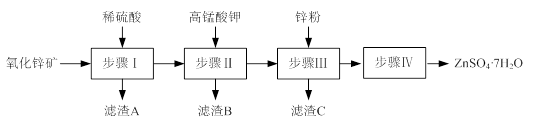
�Ķ��쳵ϵ�д���15�֣�ij�о�С���û�ͭ����Ҫ�ɷ���CuFeS2������SΪ-2�ۣ�Ϊ��Ҫԭ����ͭ�����ܷ�ӦΪ��2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2����ʵ�ϸ÷�Ӧ�ǰ��������̷ֲ����еģ�

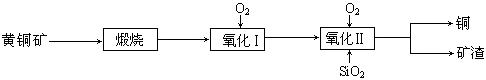
��1��������ķ�Ӧ��Ҫ���������ɵ�����������һ������Ϊ��������������������跴Ӧ���ɿ�������������Ҫ�ɷ��� ���ѧʽ����
��2���ݱ�������һ��ϸ�������������¿��Խ���ͭ�������������Σ���Ӧ����������Һ�з����ġ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3���ҹ�ѧ���о����֣��Ծ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ����������ȴ���ܽ⡢�������ᾧ���õ�CuSO4��5H2O�������ɱ��ܹ��������ࡣ�й�ʵ�������±���
| ����¯�¶�/�� | 560 | 580 | 600 | 620 | 640 | 660 |
| ˮ����Cu/% | 90.12 | 91.24 | 93.50 | 92.38 | 89.96 | 84.23 |
| ������Cu/% | 92.00 | 93.60 | 97.08 | 97.82 | 98.16 | 98.19 |
��ʵ�����������з���¯�¶�Ϊ600��620 �档������¶ȵķ����� ��
�۵��¶ȸ���600��620 ��ʱ����������ˮ����ͭ�½���ԭ���� ��
����������ȴ��ij�����ʵ�����������Ҫ�� ����֪����Һ�У�Cu2+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.7��6.7��Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.1��3.2������Ƶõ�����ͭ��Һ�к���������Fe3+����д����ȥ��Һ��Fe3+��ʵ��������裺 ��
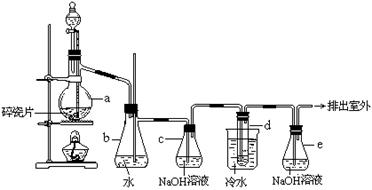
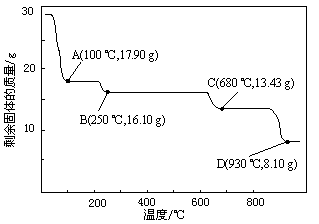
��18�֣��ס��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ����������ʵ�����̣�
ʵ���У������Ƶõİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ �ã�������������ͭ����Ӧ��ɺ�ɫ����ͭת��Ϊ��ɫ��ͭ����ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��
��С���ã���Ӧǰ����ͭ������m1 g������ͭ��Ӧ��ʣ����������m2g�����ɵ����ڱ�״���µ����V1L����С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������Ϊm4g�����ɵ����ڱ�״���µ����V2L����ش��������⣺
��1�����Aװ�������ԵIJ�����____________________________________________________��
��2��ʵ���Ҽ��鰱���IJ�����������____________________________________��
��3���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ��Ʊ�ԭ����д���±��Ŀո��С�
| | ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� |
| ��С�� | A | �������ơ������ | ��Ӧ�Ļ�ѧ����ʽΪ____________ |
| ��С�� | _____ | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã�_______ |
��5����С�����������ݼ�����������е������ԭ�Ӹ���֮������С������ֵ����ԭ����_______��Ϊ�ˣ���С����ԭ��ʵ��Ļ�����������һ��װ��ijҩ����ʵ������������ʵ�顣����ʵ��ǰ���ҩƷ�������仯�����ɵ�����������ó��˺�����ʵ��������ҩƷ��������________��
[14��]ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
�Ʊ������л���
 �ȸ���Ӧ��
�ȸ���Ӧ��
��Ҫʵ��װ�úͲ������£�
��I���ϳɣ�������ƿ�м���20 g��ˮAlCl3��30 mL��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6 mL��������10 mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ�����������μ���Ϻ���Ȼ���1Сʱ��
���������ᴿ��
�ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л���
��ˮ���ñ���ȡ����Һ
�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ
������ֲ�Ʒ�õ�����ͪ���ش��������⣺
��1������a�����ƣ�____________��װ��b�����ã�________________________________��
��2���ϳɹ�����Ҫ����ˮ������������____________________________________________��
��3�������������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���_________________��
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |
��5����Һ©��ʹ��ǰ��___________________��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ��________________����Һ©����������̨�������Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ��________________��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ�������6���ֲ�Ʒ�����ᴿʱ������װ�����¶ȼ�λ����ȷ����________________�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����________________��



 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺