��Ŀ����
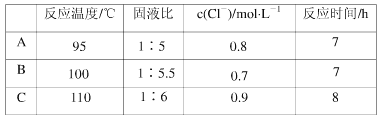
����Ŀ�������������ѧ��Fulvio Cacace���˻���˼��������о��������̬N4���ӣ�����ӽṹ����ͼ��ʾ����֪����1 mol N��N����167 kJ����������1 mol N![]() N�ų�942 kJ����������������Ϣ�����ݣ��ж�����˵����ȷ����
N�ų�942 kJ����������������Ϣ�����ݣ��ж�����˵����ȷ����

A. N4����һ�����͵Ļ�����
B. N4�����д��ڷǼ��Լ�
C. N4������N��N����Ϊ109��28��
D. 1 mol N4ת���N2������882 kJ����
���𰸡�B
��������
A��N4��N��ɣ���һ�ֵ��ʣ�A����B��N4�����д��ڵ����������ڷǼ��Լ���B��ȷ��C�����Ӳ�����������ṹ������N4������N-N���Dz���109��28����C����D��1molN4�����к���0.6molN-N����������2molN2���γ�2molN��N������1moN4����ת��ΪN2��ѧ���������յ�����Ϊ6��167kJ=1002kJ���γɻ�ѧ���ų�������Ϊ2��942kJ=1884kJ�����Է�Ӧ���ȣ��ų�������Ϊ1884kJ-1002kJ=882kJ����ӦΪ�ų�882kJ������D����ѡB��

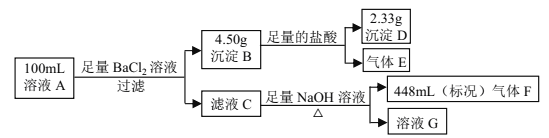
����Ŀ��ú�������Ƕ�ú������ӹ�����Ҫ����������Ҫ��Ӧ��̼��ˮ������Ӧ����ˮú����
��1����֪��101KPa��150��ʱ����1mol��̼��һ����̼���������������������ȼ�ղ��ָ���ԭ�¶ȣ����ų��������ֱ�Ϊ393.7kJ/mol��283.0kJ/mol��242.0 kJ/mol����д��ú��������Ӧ���Ȼ�ѧ����ʽ__________________���������ѧ�Ƕȼ����÷�Ӧ��ij�������ܹ�������е�ԭ����_______________��
��2���ں����ܱ�����������ʵ�����1:1����һ������̼��ˮ������Ӧ����ˮú����һ�������´ﵽƽ�⣬���ı䷴Ӧ��ijһ����ʱ�����б仯��˵��ƽ��һ��������Ӧ�����ƶ�����_________��������ţ�
A.����Ӧ�������������� B.��ѧƽ�ⳣ��K����
C.��������ƽ����Է����������� D.��Ӧ�����������������
��3��һ��������ú���������ﻹ���Է�����Ӧ��700��ʱ�����ݻ�Ϊ2L���ܱ������г���һ������CO��H2O��������Ӧ��CO(g)+H2O(g)![]() CO2(g)+ H2(g) ��Ӧ�����вⶨ�IJ������ݼ��±���
CO2(g)+ H2(g) ��Ӧ�����вⶨ�IJ������ݼ��±���
��Ӧʱ��/ min | n(CO)/ mol | n(H2O)/ mol |
0 | 1.20 | 0.60 |
5 | 0.80 | |
10 | 0.20 |
����ݱ���ش��������⣺
�ټ��㷴Ӧ��5min����������ʾ��ƽ������Ϊ��_____________��
����ʽ������÷�Ӧ��700��ʱ�Ļ�ѧƽ�ⳣ��K=__________������¶�����800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ___________������ȡ����ȡ�����Ӧ��
�۽��ϱ��д�ƽ���Ļ������ָ������£���200mL2mol/LNaOH��Һ�������գ���ַ�Ӧ�������Һ������䣬����˵������ȷ���ǣ�����֪HCO3-���볣��ԼΪ4![]() 10-11��CaCO3���ܶȻ�����ԼΪ3
10-11��CaCO3���ܶȻ�����ԼΪ3![]() 10-9��___________
10-9��___________
A.c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-��
B.c��H2CO3����c��CO32-��
C.c��Na+��+c��OH-��=c��H+��+c��HCO3-��+2c��H2CO3��
D.�����Һ�м���������Ũ�ȵ�CaCl2��Һ������ɫ����