��Ŀ����
����Ŀ��[��ѧ��ѡ��3�����ʽṹ������]ij�������о����ڹ����β��Ϻ���˸����������о���ȡ�����ش�ijɾ͡�
��1��SiO44-�ǹ��ɹ����εĻ����ṹ��Ԫ��ͨ���������������γɹ�����Ⱥ��
�ٻ�̬��ԭ�Ӽ۵����Ų�ͼ��________��
����SiO44-��Ϊ�ȵ��������������______����дһ�֣���
����ͼΪһ�ֹ�����Ⱥ�Ľṹʾ��ͼ�������ӷ���Ϊ______,����Siԭ�ӵ��ӻ���ʽΪ____��
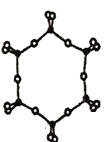
�ܵ縺�ԣ�Si______O���>������=����<��) ��
��2������Ǧ��PWO����һ����˸���壬�����ṹ��ͼ��ʾ��
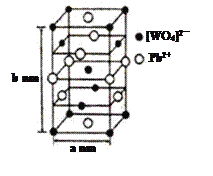
��PWO��������ṹʽʽΪ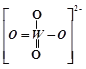 ���������Цļ��ͦм��ĸ�������______��
���������Цļ��ͦм��ĸ�������______��
��PWO�����к��е�������������______�����ţ���
A�����»��� B�����Ӽ�
C�����ۼ� D��������
�ۼ�֪�����Ħ������ΪMg��mol-1����þ�����ܶ�d=_______g��cm-3���������ӵ�������NA��ʾ��
���𰸡� ![]() PO43-��SO42-��ClO4-��CCl4 (����������Ҳ����) Si6O1812- sp3 < 2��1 BC
PO43-��SO42-��ClO4-��CCl4 (����������Ҳ����) Si6O1812- sp3 < 2��1 BC ![]()
��������(1)�ٹ�Ϊ14��Ԫ�أ���̬��ԭ�Ӽ۵��ӵĹ����ʾʽΪ![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��
��ԭ������������������ȵ�����Ϊ�ȵ����壬��SiO44-��Ϊ�ȵ����������PO43-��SO42-��ClO4-��CCl4�ȣ���ȷ�𰸣�PO43-��SO42-��ClO4-��CCl4������һ����
�۸��ݹ�����Ⱥ�Ľṹʾ��ͼ���ṹ�к���6�����������壬�����ӷ���ΪSi6O1812-������Siԭ������Χ4��Oԭ������������sp3�ӻ�����ȷ�𰸣�Si6O1812-��sp3��
��OԪ�صĵ縺�Ա�̼��̼�ĵ縺�Աȹ����˵縺�ԣ�Si��O����ȷ�𰸣�����
(2)��PWO��������ṹʽΪ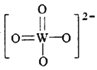 ���������У�����2��W-O����2��W=O����˦Ҽ��ͦм��ĸ�����Ϊ4��2=2:1����ȷ�𰸣�2:1��
���������У�����2��W-O����2��W=O����˦Ҽ��ͦм��ĸ�����Ϊ4��2=2:1����ȷ�𰸣�2:1��
�ڸ���PWO�����к����������ӣ��������ӽ��壬�������Ӽ������������к��й��ۼ�����ȷѡ��BC��
����1�������к��������ӵ���ĿΪ8��![]() +4��
+4��![]() +1=4�����������ӵ���ĿΪ4��
+1=4�����������ӵ���ĿΪ4��![]() +6��
+6��![]() =4,1mol����������Ϊ4Mg��1mol���������Ϊ(a��10-7cm)2��b��10-7cm��NA=NAa2b��10-21cm3����þ�����ܶ�d=4Mg��NAa2b��10-21cm3=
=4,1mol����������Ϊ4Mg��1mol���������Ϊ(a��10-7cm)2��b��10-7cm��NA=NAa2b��10-21cm3����þ�����ܶ�d=4Mg��NAa2b��10-21cm3=![]() g��cm-3����ȷ�𰸣�
g��cm-3����ȷ�𰸣�![]() ��
��