��Ŀ����
��1L�ܱ�����������Ӧ��4NH3(g)��5O2(g) 4NO(g)��6HO(g) ��H����Q kJ�� mol��1(Q��0)�������ڲ������ʵ����ʵ���Ũ�����±���
4NO(g)��6HO(g) ��H����Q kJ�� mol��1(Q��0)�������ڲ������ʵ����ʵ���Ũ�����±���
| ʱ�䣯Ũ�� | c(NH3)( mol��L-1) | c(O2)( mol��L-1) | c(NO)( mol��L-1) |
| ��ʼ | 0.8 | 1.6 | 0 |
| ��2min | 0.6 | a | 0.2 |
| ��4min | 0.3 | 0.975 | 0.5 |
| ��6min | 0.3 | 0.975 | 0.5 |
| ��8min | 0.7 | 1.475 | 0.1 |
| ��10min | 0.7 | 1.475 | 0.1 |
A����Ӧ�ڵ�2min����4minʱ��O2��ƽ������Ϊ0.1875 mol��L-1��min-1
B����Ӧ�ڵ�2minʱ�ı���ijһ������������������ʹ�ô����������¶�
C����4min����8minʱ�ֱ�ﵽ��ѧƽ�⣬��ƽ�ⳣ����ͬ
D���ڿ�ʼ��Ӧ��ǰ2min�ڣ��÷�Ӧ�ų�0.05QKJ������
C
�������������A. ��Ӧ�ڵ�2min����4minʱV(NH3)=(0.6-0.3)mol/L��2min=0.15mol/(L��min);V(O2)=" 5/4" V(NH3)= 5/4��0.15mol/(L��min)=0.1875mol/(L��min).��ȷ��B����Ӧ�ӿ�ʼ��2minʱ��c(NH3)=0.2mol/L,���Ը��ݷ���ʽ���������Ĺ�ϵ��������Ũ�ȸı���ֵΪ5/4��0.2mol/L=0.25mol/L�����a=1.35mol/L.�ڵ�2minʱ�ı���ijһ������ʹ��Ӧ���ʴ��ӿ졣�ı������������ʹ�ô����������¶ȡ���ȷ��C����4min�Ļ�ѧƽ�ⳣ��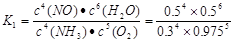 ����8minʱ�ﵽ��ѧƽ���ƽ�ⳣ��
����8minʱ�ﵽ��ѧƽ���ƽ�ⳣ��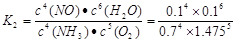 .���߶�����ֵ����ȡ�����D���ڿ�ʼ��Ӧ��ǰ2min�ڣ�NH3������Ӧ�����ʵ���Ϊ0.2mol.�����Ȼ�ѧ����ʽ��֪���÷�Ӧ�ų�0.05QKJ����������ȷ��
.���߶�����ֵ����ȡ�����D���ڿ�ʼ��Ӧ��ǰ2min�ڣ�NH3������Ӧ�����ʵ���Ϊ0.2mol.�����Ȼ�ѧ����ʽ��֪���÷�Ӧ�ų�0.05QKJ����������ȷ��
���㣺���黯ѧ��Ӧ���ʡ���ѧƽ�ⳣ������Ӧ�ȵļ��㼰Ӱ�컯ѧƽ������ص�֪ʶ��

 ������������ϵ�д�
������������ϵ�д���֪��Ӧ4CO��2NO2 N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ���������ǣ� ��
N2��4CO2�ڲ�ͬ�����µĻ�ѧ��Ӧ�������£����б�ʾ��Ӧ���������ǣ� ��
| A��v(CO)��1.5 mol��L��1��min��1 | B��v(NO2)��0.7 mol��L��1��min��1 |
| C��v(N2)��0.4 mol��L��1��min��1 | D��v(CO2)��1.1 mol��L��1��min��1 |
mA(g)+nB(g) 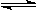 pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� ��
pC(g)+qQ(g)��m��n��p��qΪ��������ʱ���ﵽƽ��ı�־�ǣ� ��
����ϵ��ѹǿ���ٸı� �ھ�����ϵ���¶Ȳ��ٸı� �۸���ֵ�Ũ�Ȳ��ٸı�
�ܸ���ֵ������������ٸı� �ݷ�Ӧ����vA: vB: vC: vD��=��m:n:p:q
��λʱ����m mol A�ϼ���Ӧ��ͬʱp mol CҲ�ϼ���Ӧ
| A���ۢܢݢ� | B���ڢۢܢ� | C���٢ۢܢ� | D���٢ۢܢ� |
��ͼ�ǹ��ڷ�ӦA2(g)+3B2(g) 2C(g)(����ӦΪ���ȷ�Ӧ)��ƽ���ƶ�ͼ��Ӱ��ƽ���ƶ���ԭ������ǣ� ��
2C(g)(����ӦΪ���ȷ�Ӧ)��ƽ���ƶ�ͼ��Ӱ��ƽ���ƶ���ԭ������ǣ� ��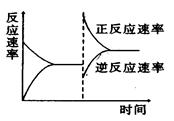
| A�������¶ȣ�ͬʱ��ѹ | B�������¶ȣ�ͬʱ��ѹ |
| C������Ӧ��Ũ�ȣ�ͬʱ��С������Ũ�� | D������Ӧ��Ũ�ȣ�ͬʱʹ�ô��� |
�����¶ȣ��������ݲ�һ��ͬʱ������� ( )
| A����ѧ��Ӧ����v | B��������ʵĵ���ƽ�ⳣ��Ka |
| C����ѧƽ�ⳣ��K | D��ˮ�����ӻ�����KW |
X��Y��ZΪ�������壬��a mol X��b mol Y����һ�ܱ������У�������ӦX��2Y 2Z���ﵽƽ��ʱ�������ǵ����ʵ������㣺n(X)��n(Y)��n(Z)����Y��ת����Ϊ (����)
2Z���ﵽƽ��ʱ�������ǵ����ʵ������㣺n(X)��n(Y)��n(Z)����Y��ת����Ϊ (����)
A�� ��100% ��100% | B�� ��100% ��100% | C�� ��100% ��100% | D�� ��100% ��100% |
���й��ڻ�ѧ��Ӧ���ʵ�˵������ȷ����( )
| A��������ѧ��Ӧ���ʵ���Ҫ�������¶� |
| B����Ӧ�������ں�����ѧ��Ӧ���п��� |
| C�����淴Ӧ�ﵽƽ��״̬ʱ���淴Ӧ�����ʶ�Ϊ0 |
| D������Ӧ���������߷�Ӧ�¶�һ��������Ӧ���� |
���ڿ��淴Ӧ2SO2+O2 2SO3���ڻ�������г���һ������18O2���㹻����ʱ���18Oԭ�� �� ��
2SO3���ڻ�������г���һ������18O2���㹻����ʱ���18Oԭ�� �� ��
| A��ֻ������O2�� | B��ֻ������O2��SO3�� |
| C��ֻ������O2��SO2�� | D��������O2��SO2��SO3�� |
�����й�˵����ȷ����
| A��CaCO3(s) =CaO(s)+CO2(g)�����²����Է����У�˵���÷�Ӧ�ġ�H<0 |
| B����ͭ����Ʒ�Ʋ����������Ʒ������ǰ���������� |
| C��N2(g)+3H2(g) =2NH3(g) ��H<0��������������ʱ�����¶ȣ���Ӧ����v(H2 )��H2��ƽ��ת���ʾ����� |
| D��ˮ�����ӻ�����Kw �����¶ȵ����߶�����˵��ˮ�ĵ����Ƿ��ȷ�Ӧ |