��Ŀ����
( 15 �֣�
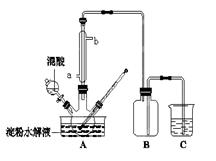
ʵ���ҳ���MnO2��Ũ���ᷴӦ�Ʊ�Cl2������װ����ͼ��ʾ����
(1)�Ʊ�ʵ�鿪ʼʱ���ȼ��װ�������ԣ��������IJ��������ǣߣ�����ţ�
A.����ƿ�м���MnO2��ĩ
B.����
C.����ƿ�м���Ũ����
(2)�Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ�������ʵ�鷽����
������������AgNO3��Һ��Ӧ���������ɵ�AgCl������
�ҷ�������������к͵ζ����ⶨ��
������������֪��CaCO3����������Ӧ������ʣ���CaCO3������
��������������Zn ��Ӧ���������ɵ�H2�����
�̶����������жϺ�ʵ�飺
�� ����������������� ��
�� �����ҷ���ʵ�飺ȷ��ȡ������Һϡ��һ����������Ϊ������
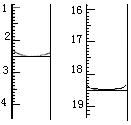
a.��ȡ����20.00 mL����0 . 1000 mol��L-1 NaOH����Һ�ζ�������22.00mL���ôεζ��������������Ũ��Ϊ mol��L-1
b.ƽ�еζ�����ʵ������
�� �жϱ�������ʵ���� ���ƫ����ƫС����ȷ������
����֪��Ksp��CaCO3 ) = 2.8��10-9��Ksp��MnCO3 ) = 2.3��10-11
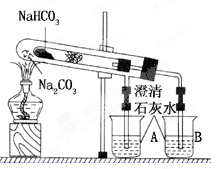
�� ���ж�����ʵ�飺װ����ͼ��ʾ���г���������ȥ����
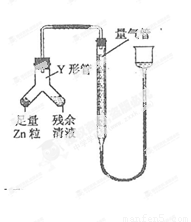
(i) ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ�����ǽ� ת�Ƶ� �С�
(ii)��Ӧ��ϣ�ÿ���1 ���Ӷ�ȡ������������������μ�С��ֱ�����䡣���������μ�С��ԭ���ǣߣ��ų�������ʵ�������Ӱ�����أ���
��1��ACB������д����� ��2���ٲ�����Һ�У�n(Cl-)��n(H+)(������������)
�� 0.1100 �� ƫС �� ������Zn�� ������Һ������д����� ������ װ����������δ��������
����������1��ע���ҩƷʱ�ȼ������MnO2����ͨ����Һ©������Ũ���ᣬ�����ܼ��ȡ�
������˳����ACB
(2)�ٸ��ݷ�Ӧ�����ӷ���ʽ��MnO2+4H��+2Cl�� Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
�ڸ���c(����)��V(����)��c(��������)��V(��������)��c(����)��c(��������)��V(��������)/ V(����)=22.00mL��0.1000 mol��L��1/20.00mL=0.1100 mol��L��1��
������KSP(MnCO3)<KSP(CaCO3)��������CaCO3Ҫת��Ϊһ����MnCO3������M(MnCO3)>M(CaCO3)��������ʣ��Ĺ����������ӣ����²�õ�c(H��)ƫС��
��Zn�����ᷴӦ���ȣ���ˣ���ȴ��������������С��
���㶨λ�����⿼���˻�ѧʵ�鷽������������ʵ�����������۵ȣ����ڿ���ѧ����ʵ�����������ݴ���������

 ��У����ϵ�д�
��У����ϵ�д�(15��)ij��ҵ��ˮ�н����±������е�5�֣�������ˮ�ĵ��뼰���ӵ�ˮ�⣩���Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol/L��
| ������ | K+ Cu2+ Fe3+ Al3+ Fe2+ |
| ������ | Cl- CO32- NO3- SO42- SiO32- |
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ������Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䡣
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
��1���ɢ��жϣ���Һ��һ�������е��������� ��д���ӷ��ţ���
��2�����м�����������������ɫ����ĵ����ӷ���ʽ��_________________________��
��3�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ
_______________________________________________________________
��4����ͬѧ����ȷ��ԭ��Һ�������������� ���������� ����д���ӷ��ţ�
��5����ȡ100mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ g��
��6����ҵ��ˮ�г����в�ͬ���͵���Ⱦ��ɲ��ò�ͬ�ķ�����������������ͬѧ��Ժ���ͬ��Ⱦ��ķ�ˮ����Ĵ�����ʩ�ͷ�����������ȷ����
| ѡ�� | ��Ⱦ�� | ������ʩ | ������� |
| A | ���� | ����ʯ���к� | ������ |
| B | Cu2+���ؽ������� | �������γ��� | ��ѧ�� |
| C | �������л���ķ�ˮ | ͨ�������л | ������ |
| D | ���Եķ�ˮ | ��CO2���к� | ��ѧ�� |