ЬтФПФкШн
Х№дЊЫидкЛЏбЇжагаКмживЊЕФЕиЮЛЃЌХ№МАЦфЛЏКЯЮяЙуЗКгІгУгкгРДХВФСЯЁЂГЌЕМВФСЯЁЂИЛШМСЯВФСЯЁЂИДКЯВФСЯЕШИпаТВФСЯСьгђЁЃ
ЃЈ1ЃЉШ§ЗњЛЏХ№дкГЃЮТГЃбЙЯТЮЊОпгаДЬБЧЖёГєКЭЧПДЬМЄадЕФЮоЩЋгаЖОИЏЪДадЦјЬхЃЌЦфЗжзгЕФСЂЬхЙЙаЭЮЊ________ЃЌBдзгЕФдгЛЏРраЭЮЊ________ЁЃ
ЃЈ2ЃЉСзЛЏХ№ЪЧвЛжжЪмЕНИпЖШЙизЂЕФФЭФЅЭПСЯЃЌЫќПЩгУзїН№ЪєЕФБэУцБЃЛЄВуЁЃЭМЃЈaЃЉЪЧСзЛЏХ№ОЇЬхЕФОЇАћЪОвтЭМЃЌдђСзЛЏХ№ЕФЛЏбЇЪНЮЊ________ЃЌИУОЇЬхЕФОЇЬхРраЭЪЧ________ЁЃ
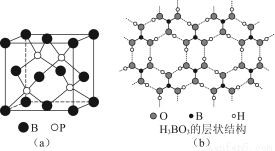
ЃЈ3ЃЉе§Х№ЫсЃЈH3BO3ЃЉЪЧвЛжжЦЌВузДНсЙЙАзЩЋОЇЬхЃЌВуФкЕФH3BO3ЗжзгМфЭЈЙ§ЧтМќЯрСЌ[ШчЭМЃЈbЃЉ]ЁЃ
ЂйХ№ЫсЗжзгжаBзюЭтВуга________ИіЕчзгЃЌ1 mol H3BO3ЕФОЇЬхжага________molЧтМќЁЃ
ЂкХ№ЫсШмгкЫЎЩњГЩШѕЕчНтжЪвЛЫЎКЯХ№ЫсBЃЈOHЃЉ3ЁЄH2OЃЌЫќЕчРыЩњГЩЩйСП[BЃЈOHЃЉ4]ЃКЭHЃЋРызгЁЃдђХ№ЫсЮЊ________дЊЫсЃЌ[BЃЈOHЃЉ4]ЃКЌгаЕФЛЏбЇМќРраЭЮЊ________ЁЃ
ЃЈ1ЃЉЦНУцШ§НЧаЮЁЁsp2
ЃЈ2ЃЉBPЁЁдзгОЇЬх
ЃЈ3ЃЉЂй6ЁЁ3ЁЁЂквЛЁЁЙВМлМќЁЂХфЮЛМќ
ЁОНтЮіЁПЃЈ1ЃЉBF3ЗжзгжаЃЌBдзгаЮГЩСЫ3ИіІвМќЃЌВЛКЌЙТЕчзгЖдЃЌЙЪдгЛЏЙьЕРЪ§ЮЊ3ЃЌдгЛЏЗНЪНЮЊsp2дгЛЏЃЌBF3ЗжзгСЂЬхЙЙаЭЮЊЦНУцШ§НЧаЮЁЃЃЈ2ЃЉвЛИіОЇАћжаКЌгаBдзгЕФИіЪ§ЮЊ8ЁС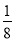 ЃЋ6ЁС
ЃЋ6ЁС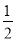 ЃН4ИіЃЌPдзгИіЪ§ЮЊ4ИіЃЌЙЪЛЏбЇЪНЮЊBPЃЛСзЛЏХ№ОЇЬхжажЛКЌЙВМлМќЧвФЭФЅЃЌЮЊдзгОЇЬхЁЃЃЈ3ЃЉЂйгЩЭМПЩжЊЃЌBдзгаЮГЩСЫШ§ИіЙВМлЕЅМќЃЌзюЭтВуЕчзгЪ§ЮЊ6ЃЛ1 mol H3BO3ЕФОЇЬхжаКЌга3 mol HдзгЃЌУПИіHдзгЖМгыЯрСкЕФOдзгаЮГЩЧтМќЃЌЙЪЧтМќИіЪ§ЮЊ3 molЃЛЂк1 mol BЃЈOHЃЉ3ЁЄH2OПЩЕчРыГі1 mol HЃЋЃЌЙЪХ№ЫсЮЊвЛдЊЫсЃЛ[BЃЈOHЃЉ4]ЃРызгжаBдзггавЛИіПеЕФ2pЙьЕРЃЌЖјOHЃКЌгаЙТЕчзгЖдЃЌСНепжЎМфПЩвдаЮГЩХфЮЛМќЁЃ
ЃН4ИіЃЌPдзгИіЪ§ЮЊ4ИіЃЌЙЪЛЏбЇЪНЮЊBPЃЛСзЛЏХ№ОЇЬхжажЛКЌЙВМлМќЧвФЭФЅЃЌЮЊдзгОЇЬхЁЃЃЈ3ЃЉЂйгЩЭМПЩжЊЃЌBдзгаЮГЩСЫШ§ИіЙВМлЕЅМќЃЌзюЭтВуЕчзгЪ§ЮЊ6ЃЛ1 mol H3BO3ЕФОЇЬхжаКЌга3 mol HдзгЃЌУПИіHдзгЖМгыЯрСкЕФOдзгаЮГЩЧтМќЃЌЙЪЧтМќИіЪ§ЮЊ3 molЃЛЂк1 mol BЃЈOHЃЉ3ЁЄH2OПЩЕчРыГі1 mol HЃЋЃЌЙЪХ№ЫсЮЊвЛдЊЫсЃЛ[BЃЈOHЃЉ4]ЃРызгжаBдзггавЛИіПеЕФ2pЙьЕРЃЌЖјOHЃКЌгаЙТЕчзгЖдЃЌСНепжЎМфПЩвдаЮГЩХфЮЛМќЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИбаОПCO2ЕФРћгУЖдДйНјЕЭЬМЩчЛсЕФЙЙНЈОпгаживЊЕФвтвхЁЃ
(1)НЋCO2гыНЙЬПзїгУЩњГЩCOЃЌCOПЩгУгкСЖЬњЕШЁЃ
ЂйвбжЊЃКFe2O3(s)ЃЋ3C(ЪЏФЋ)=2Fe(s)ЃЋ3CO(g)ЁЁІЄH1ЃНЃЋ489.0 kJЁЄmolЃ1
C(ЪЏФЋ)ЃЋCO2(g)=2CO(g)ЁЁІЄH2ЃНЃЋ172.5 kJЁЄmolЃ1
дђCOЛЙдFe2O3ЕФШШЛЏбЇЗНГЬЪНЮЊ___________________________
ЂкРћгУШМЩеЗДгІПЩЩшМЦГЩCO/O2ШМСЯЕчГи(вдKOHШмвКЮЊЕчНтвК)ЃЌаДГіИУЕчГиЕФИКМЋЗДгІЪН___________________________________________
(2)ФГЪЕбщНЋCO2КЭH2ГфШывЛЖЈЬхЛ§ЕФУмБеШнЦїжаЃЌдкСНжжВЛЭЌЬѕМўЯТЗДгІЃК
CO2(g)ЃЋ3H2(g) CH3OH(g)ЃЋH2O(g)ЁЁ ІЄHЃНЃ49.0 kJЁЄmolЃ1
CH3OH(g)ЃЋH2O(g)ЁЁ ІЄHЃНЃ49.0 kJЁЄmolЃ1
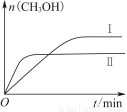
ВтЕУCH3OHЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЩЯЭМЫљЪОЃЌЛиД№ЮЪЬтЃК
ЂйЯТСаДыЪЉжаФмЪЙn(CH3OH)/n(CO2)діДѓЕФЪЧ________ЁЃ
AЃЎЩ§ИпЮТЖШ BЃЎГфШыHe(g)ЪЙЬхЯЕбЙЧПдіДѓ
CЃЎНЋH2O(g)ДгЬхЯЕжаЗжРы DЃЎдйГфШы1 mol CO2КЭ3 mol H2
ЂкЧњЯпЂёЁЂЂђЖдгІЕФЦНКтГЃЪ§ДѓаЁЙиЯЕЮЊKЂё________KЂђ(ЬюЁАДѓгкЁБЁАЕШгкЁБЛђЁАаЁгкЁБ)ЁЃ
ЂлвЛЖЈЮТЖШЯТЃЌдкШнЛ§ЯрЭЌЧвЙЬЖЈЕФСНИіУмБеШнЦїжаЃЌАДШчЯТЗНЪНЭЖШыЗДгІЮяЃЌвЛЖЮЪБМфКѓДяЕНЦНКтЁЃ
ШнЦї | Мз | вв |
ЗДгІЮяЭЖШыСП | 1 mol CO2ЁЂ3 mol H2 | a mol CO2ЁЂb mol H2ЁЂc mol CH3OH(g)ЁЂc mol H2O(g) |
ШєМзжаЦНКтКѓЦјЬхЕФбЙЧПЮЊПЊЪМЪБЕФ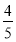 ЃЌвЊЪЙЦНКтКѓввгыМзжаЯрЭЌзщЗжЕФЬхЛ§ЗжЪ§ЯрЕШЃЌЧвЦ№ЪМЪБЮЌГжЗДгІФцЯђНјааЃЌдђcЕФШЁжЕЗЖЮЇЮЊ________ЁЃ
ЃЌвЊЪЙЦНКтКѓввгыМзжаЯрЭЌзщЗжЕФЬхЛ§ЗжЪ§ЯрЕШЃЌЧвЦ№ЪМЪБЮЌГжЗДгІФцЯђНјааЃЌдђcЕФШЁжЕЗЖЮЇЮЊ________ЁЃ
(3)гУ0.10 molЁЄLЃ1бЮЫсЗжБ№ЕЮЖЈ20.00 mL 0.10 molЁЄLЃ1ЕФNaOHШмвККЭ20.00 mL 0.10 molЁЄLЃ1АБЫЎЫљЕУЕФЕЮЖЈЧњЯпШчЯТЃК
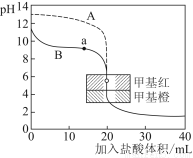
ЧыжИГібЮЫсЕЮЖЈАБЫЎЕФЧњЯпЮЊ________(ЬюЁАAЁБЛђЁАBЁБ)ЃЌЧыаДГіЧњЯпaЕуЫљЖдгІЕФШмвКжаИїРызгХЈЖШгЩДѓЕНаЁЕФХХСаЫГађ________ЁЃ