��Ŀ����
X��Y��Z������Ԫ�صĵ��ʣ�K��H�ǻ�������������¹�ϵ��ʽ�и����ʵĻ�ѧ�������ͷ�Ӧ��������ȥ����
��1����X+K H+Y ��X+Z
H+Y ��X+Z H ��Y+Z
H ��Y+Z K
K
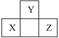
��X��Y�����Ԫ����ͬһ���壬��д�����Ϣ�ʽ��������ͬ�Ļ�ѧ����ʽ������ƽ��___________________�� __________________��__________________��
��X��Y�����Ԫ�ز���ͬһ���壬��д�����Ϣ�ʽ��3����ͬ�Ļ�ѧ����ʽ������ƽ��3��ʽ���е�3��X��3��Y���ֱ��ɲ�ͬ��Ԫ����ɣ���__________��__________��__________��
��2������ij������W������������K��CO2��Ӧ���������ɵ���Y�����ϸ������������3����ͨʽ�����ĵ���X��__________������Y��__________������Z��__________��������W��__________������д����ѧʽ��
��1����X+K
 H+Y ��X+Z
H+Y ��X+Z H ��Y+Z
H ��Y+Z K
K��X��Y�����Ԫ����ͬһ���壬��д�����Ϣ�ʽ��������ͬ�Ļ�ѧ����ʽ������ƽ��___________________�� __________________��__________________��
��X��Y�����Ԫ�ز���ͬһ���壬��д�����Ϣ�ʽ��3����ͬ�Ļ�ѧ����ʽ������ƽ��3��ʽ���е�3��X��3��Y���ֱ��ɲ�ͬ��Ԫ����ɣ���__________��__________��__________��
��2������ij������W������������K��CO2��Ӧ���������ɵ���Y�����ϸ������������3����ͨʽ�����ĵ���X��__________������Y��__________������Z��__________��������W��__________������д����ѧʽ��
��1��2Na+2H2O====2NaOH+H2��
O2+2H2S====2S��+2H2O
2C+SiO2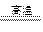 Si+2CO��
Si+2CO��
2F2+2H2O====4HF+O2
Cl2+H2S====S��+2HCl
3O2+4NH3====2N2+6H2O
(2)F2 O2 H2 Na2O2
O2+2H2S====2S��+2H2O
2C+SiO2
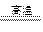 Si+2CO��
Si+2CO��2F2+2H2O====4HF+O2
Cl2+H2S====S��+2HCl
3O2+4NH3====2N2+6H2O
(2)F2 O2 H2 Na2O2
��1��Ҫд�����ϵڣ�1��С���е�����ͬһ����Ļ�ѧ����ʽ�����ȷ�����ʽ�����û���Ӧ������ͬһ������û���Ӧ�ķ���ʽ�У�2Na+2H2O====2NaOH+H2����O2+2H2S====2S��+2H2O;SiO2+2C====Si+2CO����Ҫд�����Ϣ�ʽ�IJ���ͬһ������û���Ӧ�Ļ�ѧ����ʽ����Ҫ��ϵڣ�2��С��ȷ��K��H2O��Y��O2��Z��H2���ٸ��ݵ��ʸ�ˮ��Ӧ���������ɣ�ȷ��X��F2����˷��Ϣ�ʽ�Ļ�ѧ����ʽ�У�2F2+2H2O====4HF+O2��Cl2+H2S====S��+2HCl;4NH3+3O2====2N2+6H2O��
��2���ɵڣ�1��С��������̿�֪����X��F2������Y��O2������Z��H2��������W��Na2O2��
��2���ɵڣ�1��С��������̿�֪����X��F2������Y��O2������Z��H2��������W��Na2O2��

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
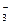 ��Cl-
��Cl-