̀âÄ¿ÄÚÈƯ
¡¾̀âÄ¿¡¿Ä³ÑĐ¾¿Đ¡×éΪÁË̀½¾¿̉»ÖÖÎ̃»ú¿óÎïÑÎX£¨½öº¬ËÄÖÖÔªËØ£¬Ç̉Ϊ¸´ÑΣ©µÄ×é³ÉºÍĐÔÖÊ£¬Éè¼Æ²¢Íê³ÉÁËÈçÏÂʵÑ飨עaq±íʾÈÜ̉º£©£º 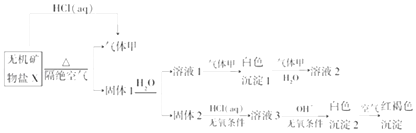
È¡10.80g XÔÚ¶èĐÔÆøÁ÷ÖĐ¼ÓÈÈÖÁÍêÈ«·Ö½â£¬µĂµ½6.40g¹̀̀å1ºÍ0.1molµÄÆø̀å¼×£®»Ø´đÈçÏÂÎỀ⣺
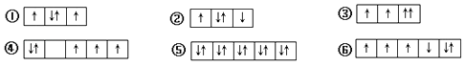
£¨1£©»³ö°×É«³Áµí1ÖĐƠư¶₫¼Û½đÊôÔªËصÄÀë×ӽṹʾ̉âͼ £¬ Đ´³öÆø̀å¼×µÄµç×Óʽ £®
£¨2£©XµÄ»¯Ñ§Ê½ÊÇ £¬ ÔÚ¶èĐÔÆøÁ÷ÖĐ¼ÓÈÈXÖÁÍêÈ«·Ö½âµÄ»¯Ñ§·½³̀ʽΪ £®
£¨3£©°×É«³Áµí2ÔÚ¿ƠÆøÖбä³Éº́ºÖÉ«³ÁµíµÄỘ̉ÊÇ£¨ÓĂ»¯Ñ§·½³̀ʽ±íʾ£©£®
£¨4£©̉»¶῭ơ¼₫Ï£¬Æø̀å¼×Óë¹̀̀å1ÖĐµÄ³É·Ö¿ÉÄÜ·¢ÉúÑơ»¯»¹Ô·´Ó¦£®
¡¾´đ°¸¡¿
£¨1£©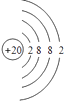 £»
£»![]()
£¨2£©CaFe£¨CO3£©2£»CaFe£¨CO3£©2 ![]() CaO+FeO+2CO2¡ü
CaO+FeO+2CO2¡ü
£¨3£©4Fe£¨OH£©2+2H2O+O2=4Fe£¨OH£©3
£¨4£©FeO
¡¾½âÎö¡¿½â£º£¨1£©¸ù¾ỬÔÉÏ·ÖÎö£¬°×É«³Áµí1ÊÇCaCO3 £¬ ̀¼Ëá¸ÆÖĐ½đÊôÔªËصÄÔ×ÓºËÍâÓĐ4¸öµç×Ó²ă¡¢×îÍâ²ăÓĐ2¸öµç×Ó£¬Ëù̉ÔCaÔ×ӽṹʾ̉âͼΪ 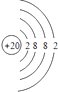 £¬Æø̀å¼×ÊǶ₫Ñơ»¯̀¼£¬¶₫Ñơ»¯̀¼µÄµç×ÓʽΪ
£¬Æø̀å¼×ÊǶ₫Ñơ»¯̀¼£¬¶₫Ñơ»¯̀¼µÄµç×ÓʽΪ ![]() £¬Ëù̉Ô´đ°¸ÊÇ£º
£¬Ëù̉Ô´đ°¸ÊÇ£º  £»
£» ![]() £»£¨2£©Í¨¹ửÔÉÏ·ÖÎöÖª£¬XµÄ»¯Ñ§Ê½ÊÇ CaFe£¨CO3£©2 £¬ ÔÚ¶èĐÔÆøÁ÷ÖĐ¼ÓÈÈXÖÁÍêÈ«·Ö½âÉú³É¶₫Ñơ»¯̀¼ºÍÑơ»¯¸Æ¡¢Ñơ»¯ÑÇ̀ú£¬Ëù̉Ը÷´Ó¦µÄ»¯Ñ§·´Ó¦·½³̀ʽΪCaFe£¨CO3£©2
£»£¨2£©Í¨¹ửÔÉÏ·ÖÎöÖª£¬XµÄ»¯Ñ§Ê½ÊÇ CaFe£¨CO3£©2 £¬ ÔÚ¶èĐÔÆøÁ÷ÖĐ¼ÓÈÈXÖÁÍêÈ«·Ö½âÉú³É¶₫Ñơ»¯̀¼ºÍÑơ»¯¸Æ¡¢Ñơ»¯ÑÇ̀ú£¬Ëù̉Ը÷´Ó¦µÄ»¯Ñ§·´Ó¦·½³̀ʽΪCaFe£¨CO3£©2 ![]() CaO+FeO+2CO2¡ü£¬Ëù̉Ô´đ°¸ÊÇ£ºCaFe£¨CO3£©2£»CaFe£¨CO3£©2
CaO+FeO+2CO2¡ü£¬Ëù̉Ô´đ°¸ÊÇ£ºCaFe£¨CO3£©2£»CaFe£¨CO3£©2 ![]() CaO+FeO+2CO2¡ü£»£¨3£©ÇâÑơ»¯ÑÇ̀ú²»Îȶ¨£¬̉×±»¿ƠÆøÑơ»¯Éú³Éº́ºÖÉ«ÇâÑơ»¯̀ú£¬Ëù̉Ô°×É«³Áµí2ÔÚ¿ƠÆøÖбä³Éº́ºÖÉ«³Áµí£¬·´Ó¦·½³̀ʽΪ4Fe£¨OH£©2+2H2O+O2=4Fe£¨OH£©3 £¬ Ëù̉Ô´đ°¸ÊÇ£º4Fe£¨OH£©2+2H2O+O2=4Fe£¨OH£©3£»£¨4£©̉»¶῭ơ¼₫Ï£¬Æø̀å¼×Óë¹̀̀å1ÖеÄijÖֳɷֿÉÄÜ·¢ÉúÑơ»¯»¹Ô·´Ó¦£¬Ñơ»¯ÑÇ̀ú¾ßÓĐ»¹ÔĐÔ£¬Äܱ»¶₫Ñơ»¯̀¼Ñơ»¯Éú³ÉËÄÑơ»¯Èừú»̣Ñơ»¯̀ú£¬Í¬Ê±Éú³ÉCO£¬·´Ó¦·½³̀ʽΪ2FeO+CO2
CaO+FeO+2CO2¡ü£»£¨3£©ÇâÑơ»¯ÑÇ̀ú²»Îȶ¨£¬̉×±»¿ƠÆøÑơ»¯Éú³Éº́ºÖÉ«ÇâÑơ»¯̀ú£¬Ëù̉Ô°×É«³Áµí2ÔÚ¿ƠÆøÖбä³Éº́ºÖÉ«³Áµí£¬·´Ó¦·½³̀ʽΪ4Fe£¨OH£©2+2H2O+O2=4Fe£¨OH£©3 £¬ Ëù̉Ô´đ°¸ÊÇ£º4Fe£¨OH£©2+2H2O+O2=4Fe£¨OH£©3£»£¨4£©̉»¶῭ơ¼₫Ï£¬Æø̀å¼×Óë¹̀̀å1ÖеÄijÖֳɷֿÉÄÜ·¢ÉúÑơ»¯»¹Ô·´Ó¦£¬Ñơ»¯ÑÇ̀ú¾ßÓĐ»¹ÔĐÔ£¬Äܱ»¶₫Ñơ»¯̀¼Ñơ»¯Éú³ÉËÄÑơ»¯Èừú»̣Ñơ»¯̀ú£¬Í¬Ê±Éú³ÉCO£¬·´Ó¦·½³̀ʽΪ2FeO+CO2 ![]() Fe2O3+CO£¨»̣Éú³ÉFe3O4£©£¬Ëù̉Ô´đ°¸ÊÇ£ºFeO£®
Fe2O3+CO£¨»̣Éú³ÉFe3O4£©£¬Ëù̉Ô´đ°¸ÊÇ£ºFeO£®

 ¾Ù̉»·´ÈưÆÚÄ©°Ù·Ö³å´̀¾íϵÁĐ´đ°¸
¾Ù̉»·´ÈưÆÚÄ©°Ù·Ö³å´̀¾íϵÁĐ´đ°¸