��Ŀ����
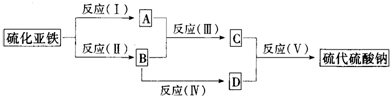
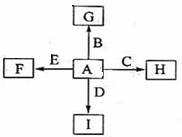
��14�֣���ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪���ٷ�ӦC+G B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��
�ش����⣺
�Ţ��з�Ӧ�Ļ�ѧ����ʽΪ���������������������������� ��
�ƻ�����I�ĵ���ʽΪ�������������� �����Ŀռ�ṹ�������������� ��
�ǽ�G�������ᣬ�õ�����Һ����һ������ͭ�ۣ�д�����ӷ���ʽ��
��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ����������������������������������������������Ӧ�����������������I��Ӧ�����ӷ���ʽΪ������������������������������������ ��
��E��I��ȼ�չ۲쵽�������������������������������������������������� ��
��1��8Al+3Fe3O4 9Fe+4Al2O3
9Fe+4Al2O3
��2��
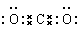 �� ֱ���� ��3�� 2Fe3++Cu=2Fe2++Cu2+
�� ֱ���� ��3�� 2Fe3++Cu=2Fe2++Cu2+
��4��2Al+2OH-+2H2O=2AlO-2+3H2��
AlO2-+CO2+2H2O=Al��OH��3��+HCO3- ��ע����Ҫ��дOH-+CO2=HCO-3��
��5��þ������ȼ�գ����ɰ�ɫ��ĩ����Ӧ���ڱڸ��к�ɫ��̼
�����������ݢٿ�֪���������ȷ�Ӧ������C��������H����������A��������G��������������B������������������������CO2������I��CO2����D��̼������CO2��ȼ�գ��ܰ�CO2�е�̼�û���������þ����E��þ��F������þ������þ��þԪ�ص�����������60����

��֪�������ݣ�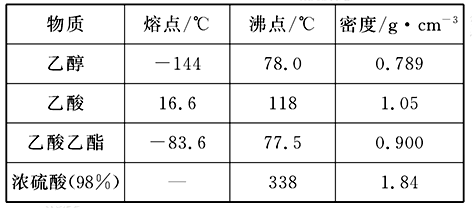
��ͼΪʵ������ȡ����������װ��ͼ��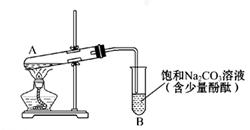
(1)������̼������Һ�Ϸ��ռ����϶�Һ��ʱ��ֹͣ���ȣ�ȡ��С�Թ�B����������á���ǰ���ʵ������_________(��ѡ��)��
| A���ϲ�Һ��䱡 | B���²�Һ���ɫ��dz���Ϊ��ɫ |
| C����������� | D���й���ζ |
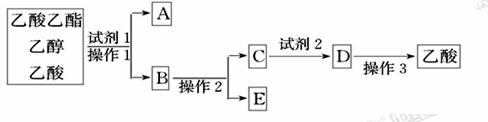
���Լ�1���ѡ��___________��
�ڲ���1��_____�����õ���Ҫ����������________��
���Լ�2���ѡ��___________��
�ܲ���2��___________��
�ݲ���3���¶ȼ�ˮ�����λ��ӦΪ����ͼ��______(��a��b��c��d)��ʾ���ڸò����У���������ƿ���¶ȼ��⣬����Ҫ�IJ���������_______��_______��_______��______���ռ�����������¶���___________��