��Ŀ����
��þ3%-5%��þ���Ͻ����ѳ�Ϊ�ִ����졢������������е�������ҵ����Ҫԭ���ϣ�����һ����֪����Ϊm1g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽����
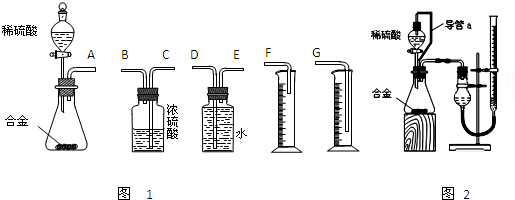
ʵ�����1��
þ���Ͻ�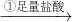 ��Һ
��Һ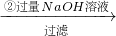 �õ�����������Ϊm2g��
�õ�����������Ϊm2g��
ʵ�����2��
þ���Ͻ�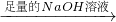 ���ɵ������ڱ�״���µ����ΪV1L��
���ɵ������ڱ�״���µ����ΪV1L��
ʵ�����3��
þ���Ͻ� ���ɵ������ڱ�״���µ����ΪV2L��
���ɵ������ڱ�״���µ����ΪV2L��
��ش��������⣺
��1��д��ʵ�����1�е�����������Ӧ�����ӷ���ʽ��______
��2��д��ʵ�����2�з�Ӧ�����ӷ���ʽ��______
��3���ж��⼸��ʵ������ܷ����þ�����������������������Ŀ�ṩ�����ݽ�þ������������ʾ��������������ľͿ��Ų��
��ʵ�����1______
��ʵ�����2______
��ʵ�����3______��
�⣺��1��ʵ�����1�е�����������Ӧ�����ӷ���ʽ�У�2Al+6H+�T2Al3++3H2��?��Al3++4OH-�T[Al��OH��4]-��Al3++3OH-�TAl��OH��3��?Al��OH��3+OH-�T[Al��OH��4]-��
�ʴ�Ϊ��2Al+6H+�T2Al3++3H2��?��Al3++4OH-�T[Al��OH��4]-��Al3++3OH-�TAl��OH��3��?Al��OH��3+OH-�T[Al��OH��4]-?����2��ʵ�����2�з��������ӷ�Ӧ�ǣ�2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2��?��
�ʴ�Ϊ��2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2��??��
��3����������þ�����ʵ���Ϊ��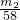 mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ��
mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ�� ��100%=
��100%=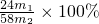 ��
��
�ʴ�Ϊ��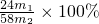 ��
��
�ڱ�״���µ����ΪV1LΪ��������������Һ��Ӧ���ɵģ����ݷ���ʽ��2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2����n��Al��=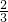 n��H2��=
n��H2��=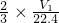 mol���Ͻ��н���������������Ϊ��
mol���Ͻ��н���������������Ϊ��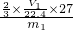 ��100%=
��100%= ��100%���Ͻ����������������ǣ�1-
��100%���Ͻ����������������ǣ�1- ��100%=
��100%= ��
��
�ʴ�Ϊ�� ��
��
����þ�����ʵ���Ϊxmol���������ʵ���Ϊymol����24x+27y=m1��
�������������������״���µ����ΪV2L��������ʽ����x+1.5y����22.4=V2��
���x=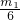 -
-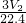 ������þ���Ͻ���þ�����������ǣ�
������þ���Ͻ���þ�����������ǣ�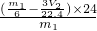 ��100%=
��100%=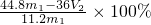 ��
��
�ʴ�Ϊ��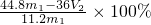 ��
��
��������1��ʵ�����1�е�����������Ӧ�У������������ᷴӦ�������Ӻ��������Ʒ�Ӧ��
��2��ʵ�����2����������������Һ��Ӧ����������ƫ�����ƣ�
��3����m2g������������þ������������þԭ���غ�����þ������������
�����ΪV1L����������������Һ��Ӧ���ɵ�������m1g�������������ʵ���������������ʵ����������þ������������
��V2L��þ�������ᷴӦ���ɵ��������������m1g�����������ʵ����г�������⼴�ɣ�
���������⿼��þ�����Ļ�ѧ���ʡ���ѧʵ�鷽������ƣ����ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����þ�����Ļ�ѧ�����ǽ���Ĺؼ���
�ʴ�Ϊ��2Al+6H+�T2Al3++3H2��?��Al3++4OH-�T[Al��OH��4]-��Al3++3OH-�TAl��OH��3��?Al��OH��3+OH-�T[Al��OH��4]-?����2��ʵ�����2�з��������ӷ�Ӧ�ǣ�2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2��?��
�ʴ�Ϊ��2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2��??��
��3����������þ�����ʵ���Ϊ��
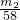 mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ��
mol������þԭ���غ㣬�Ͻ��н���þ����������Ϊ�� ��100%=
��100%=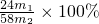 ��
���ʴ�Ϊ��
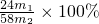 ��
���ڱ�״���µ����ΪV1LΪ��������������Һ��Ӧ���ɵģ����ݷ���ʽ��2Al+2OH-+6H2O�T2[Al��OH��4]-+3H2����n��Al��=
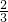 n��H2��=
n��H2��=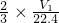 mol���Ͻ��н���������������Ϊ��
mol���Ͻ��н���������������Ϊ��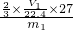 ��100%=
��100%= ��100%���Ͻ����������������ǣ�1-
��100%���Ͻ����������������ǣ�1- ��100%=
��100%= ��
���ʴ�Ϊ��
 ��
������þ�����ʵ���Ϊxmol���������ʵ���Ϊymol����24x+27y=m1��
�������������������״���µ����ΪV2L��������ʽ����x+1.5y����22.4=V2��
���x=
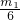 -
-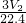 ������þ���Ͻ���þ�����������ǣ�
������þ���Ͻ���þ�����������ǣ�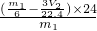 ��100%=
��100%=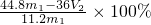 ��
���ʴ�Ϊ��
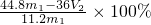 ��
����������1��ʵ�����1�е�����������Ӧ�У������������ᷴӦ�������Ӻ��������Ʒ�Ӧ��
��2��ʵ�����2����������������Һ��Ӧ����������ƫ�����ƣ�
��3����m2g������������þ������������þԭ���غ�����þ������������
�����ΪV1L����������������Һ��Ӧ���ɵ�������m1g�������������ʵ���������������ʵ����������þ������������
��V2L��þ�������ᷴӦ���ɵ��������������m1g�����������ʵ����г�������⼴�ɣ�
���������⿼��þ�����Ļ�ѧ���ʡ���ѧʵ�鷽������ƣ����ʺ����ⶨ����ѧ����ȣ��Ѷ��еȣ�����þ�����Ļ�ѧ�����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ


 ��Һ
��Һ �õ�����������Ϊm2g��
�õ�����������Ϊm2g�� ���ɵ������ڱ�״���µ����ΪV1L��
���ɵ������ڱ�״���µ����ΪV1L�� ���ɵ������ڱ�״���µ����ΪV2L��
���ɵ������ڱ�״���µ����ΪV2L��