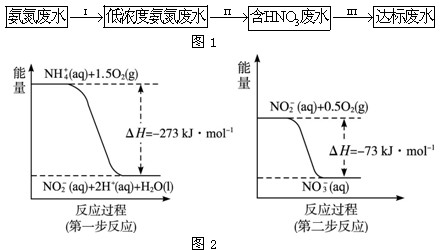
��Ŀ����
��2010?������ij�¶��£�H2��g��+CO2��g��?H2O��g��+CO��g����ƽ�ⳣ��K=
|
��������ӦH2��g��+CO2��g��?H2O��g��+CO��g��ǰ�������������䣬���º����£��ס��ҡ�����ƽ�ⳣ����ͬ���ɱ������ݿ�֪�����¶����ڼס�������������ʼŨ��n��H2����n��CO2��=1��1���ס���Ϊ��Чƽ�⣮������������ʼŨ�ȱȼ�����������ʼŨ�ȴ����ж�����̼��ת���ʱȼ��иߣ���������ʽ���ƽ�ⳣ��������������ڣ�ƽ��ʱ�����ʵ�Ũ�ȱ仯����ƽ��Ũ�ȣ�
A������������ڶ�����̼��ת���ʣ�������������ʼŨ�ȱȼ�����������ʼŨ�ȴ����ж�����̼��ת���ʱȼ��иߣ�
B���ס���Ϊ��Чƽ�⣬���кͱ���H2��ת���ʾ���ȣ���������ʽ�������������ת���ʣ�
C���ס���Ϊ��Чƽ�⣬���кͱ���CO2��ת���ʾ���ȣ���������ʽ����ƽ��ʱ���е�CO2��Ũ�ȡ�ת���ʣ������������CO2��Ũ�ȣ�
D��Ũ��Խ��Ӧ����Խ�죮
A������������ڶ�����̼��ת���ʣ�������������ʼŨ�ȱȼ�����������ʼŨ�ȴ����ж�����̼��ת���ʱȼ��иߣ�
B���ס���Ϊ��Чƽ�⣬���кͱ���H2��ת���ʾ���ȣ���������ʽ�������������ת���ʣ�
C���ס���Ϊ��Чƽ�⣬���кͱ���CO2��ת���ʾ���ȣ���������ʽ����ƽ��ʱ���е�CO2��Ũ�ȡ�ת���ʣ������������CO2��Ũ�ȣ�
D��Ũ��Խ��Ӧ����Խ�죮
����⣺���ڼ�������H2��g��+CO2��g��?H2O��g��+CO��g��
��ʼ��mol/L����0.01 0.01 0 0
�仯��mol/L����x x x x
ƽ�⣨mol/L����0.01-x 0.01-x x x
����
=
��
���x=0.006
A�������������֪���������ڶ�����̼��ת����Ϊ
��100%=60%�����º����£�������������ʼŨ�ȱȼ�����������ʼŨ�ȴ����ж�����̼��ת���ʱȼ��иߣ���ƽ��ʱ������CO2��ת���ʴ���60%����A��ȷ��
B�����º����£��ɱ������ݿ�֪�����¶����ڼס�������������ʼŨ��n��H2����n��CO2��=1��1����ӦH2��g��+CO2��g��?H2O��g��+CO��g��ǰ�������������䣬�ʼס���Ϊ��Чƽ�⣬ƽ��ʱ�����кͱ���H2��ת���ʾ���ȣ������������֪����������������ת����Ϊ
��100%=60%���ʼ��кͱ���H2��ת���ʾ�Ϊ60%����B��ȷ��
C�������������֪��ƽ��ʱ��������c��CO2��=��0.01-x ��mol/L=0.004mol/L���ס���Ϊ��Чƽ�⣬ƽ��ʱ�����кͱ���CO2��ת������ȣ���A�м����֪Ϊ60%����ƽ��ʱ��������c��CO2��=0.02mol/L����1-60%��=0.008mol/L��ƽ��ʱ������c��CO2���Ǽ��е�2������0.008mol/L����C����
D��Ũ��Խ��Ӧ����Խ�죬�ɱ������ݿ�֪���ס��������ڣ���ʼCO2Ũ����ȣ�����H2Ũ�ȱȼ���Ũ�ȴ����������ң��ף��ҡ��������ڣ���ʼH2Ũ����ȣ�����CO2Ũ�ȱ�����Ũ�ȴ��������ʱ����ң������ʱ����ң��ף���D��ȷ��
��ѡ��C��
��ʼ��mol/L����0.01 0.01 0 0
�仯��mol/L����x x x x
ƽ�⣨mol/L����0.01-x 0.01-x x x
����
| x?x |
| (0.01-x)?(0.01-x) |
| 9 |
| 4 |
���x=0.006
A�������������֪���������ڶ�����̼��ת����Ϊ
| 0.006mol/L |
| 0.01mol/L |
B�����º����£��ɱ������ݿ�֪�����¶����ڼס�������������ʼŨ��n��H2����n��CO2��=1��1����ӦH2��g��+CO2��g��?H2O��g��+CO��g��ǰ�������������䣬�ʼס���Ϊ��Чƽ�⣬ƽ��ʱ�����кͱ���H2��ת���ʾ���ȣ������������֪����������������ת����Ϊ
| 0.006mol/L |
| 0.01mol/L |
C�������������֪��ƽ��ʱ��������c��CO2��=��0.01-x ��mol/L=0.004mol/L���ס���Ϊ��Чƽ�⣬ƽ��ʱ�����кͱ���CO2��ת������ȣ���A�м����֪Ϊ60%����ƽ��ʱ��������c��CO2��=0.02mol/L����1-60%��=0.008mol/L��ƽ��ʱ������c��CO2���Ǽ��е�2������0.008mol/L����C����
D��Ũ��Խ��Ӧ����Խ�죬�ɱ������ݿ�֪���ס��������ڣ���ʼCO2Ũ����ȣ�����H2Ũ�ȱȼ���Ũ�ȴ����������ң��ף��ҡ��������ڣ���ʼH2Ũ����ȣ�����CO2Ũ�ȱ�����Ũ�ȴ��������ʱ����ң������ʱ����ң��ף���D��ȷ��
��ѡ��C��
���������黯ѧƽ����㡢��Чƽ�⡢��������Է�Ӧ���ʵ�Ӱ��ȣ�Ũ���еȣ�ע������ʽ���ⷨ�����ã��жϼס���Ϊ��Чƽ���ǽ���ؼ���

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ