��Ŀ����
����A��B��C��D���ֶ�����Ԫ�أ���֪A��Bͬ���壬B��C��Dͬ���ڣ�A��B��ԭ������֮�͵���C��D��ԭ������֮�ͣ�C�ĵ����ֱܷ��B��D������������ˮ���ﷴӦ����ش�
��1��B��C��Ԫ�ط��ŷֱ�Ϊ_____________________
��2����������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ__________________(��Ԫ�ط��ű�ʾ)
��3��D������A������ȼ�յIJ����������̼��Ӧ�Ļ�ѧ����ʽΪ_____________________
��4��B�����ܸ�D�����������ˮ�����Ũ��Һ����������ԭ��Ӧ�����ɵ������ε�ˮ��Һ���ʼ��ԣ���������ԭ��Ӧ�����ӷ���ʽΪ_____________________________
��1��B��C��Ԫ�ط��ŷֱ�Ϊ_____________________
��2����������Ԫ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ__________________(��Ԫ�ط��ű�ʾ)
��3��D������A������ȼ�յIJ����������̼��Ӧ�Ļ�ѧ����ʽΪ_____________________
��4��B�����ܸ�D�����������ˮ�����Ũ��Һ����������ԭ��Ӧ�����ɵ������ε�ˮ��Һ���ʼ��ԣ���������ԭ��Ӧ�����ӷ���ʽΪ_____________________________
��1�� S��Al ��2�� Na��Al��S��O ��3�� 2Na2O2 + 2CO2 = 2Na2CO3 + O2 ��4�� 3S + 6OH�C = 2S2�C + SO32�C + 3H2O
�������������1��C�ĵ����ֱܷ��B��D������������ˮ���ﷴӦ����֪CΪAl��D������A������ȼ�յIJ����������̼��Ӧ��D��Na��A��O������A��Bͬ���壬����BΪS��O��S��ԭ������֮��Ϊ24��Al��Na��ԭ������֮��ҲΪ24��������ȣ��ʿ���ȷ��A��B��C��D����Ԫ�طֱ�Ϊ�����������ơ���B��C��Ԫ�ط��ŷֱ�Ϊ��S��Al����2��Na��Al��Sλ��ͬ���ڣ�����ԭ������������뾶��С������Na��Al��S��O��Sλ��ͬ���壬ԭ������Խ�뾶Խ������S��O���ʴ�Ϊ��Na��Al��S��O����3��D������A������ȼ�յIJ���ΪNa2O2����CO2��Ӧ����Na2CO3��O2����Ӧ�Ļ�ѧ����ʽΪ��2Na2O2 + 2CO2 = 2Na2CO3 + O2����4����������ŨNaOH��Һ��Ӧ�����������ε�ˮ��Һ�Լ��ԣ�˵�����ɵ���������ǿ���Σ���Ԫ���γɵ�������������������ᣬ���ǵ���ӦΪ������ԭ��Ӧ�����ȷ������Na2S��Na2SO3����Ӧ�Ļ�ѧ����ʽΪ��3S + 6NaOH = 2Na2S + Na2SO3 + 3H2O����Ϊ���ӷ���ʽ��3S + 6OH�C = 2S2�C + SO32�C + 3H2O��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��Һ�������
��Һ�������
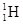 ��
��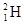 ��
��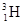 ��˵����ȷ���ǣ� ��
��˵����ȷ���ǣ� ��