��Ŀ����
�����Ƕ�ij��Һ�������Ӽ���ķ����ͽ��ۣ�������ȷ���ǣ� ��
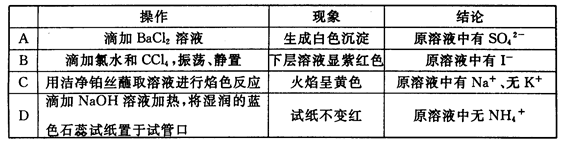
| A���ȼ���BaCl2��Һ���ټ���HNO3��Һ�������˰�ɫ����������Һ��һ�����д�����SO42�� |
| B������������CaCl2��Һ�������˰�ɫ����������Һ��һ�����д�����CO32�� |
| C���������ᣬ�ܲ���ʹ����ʯ��ˮ����ǵ����壬����Һ��һ������CO32�� |
| D���ȼ����������Ὣ��Һ�ữ���ټ���AgNO3��Һ��������ɫ����������Һ��һ�����д�����Cl�� |
D
���������A�����ܺ�SO42?�����п��ܺ���Cl?��SO32?������B�������ܺ���SO32?��PO43?�����ӣ�����C�������ܺ���HCO3?��SO32?��HSO3?�����ӣ�����D���ȼ����������Ὣ��Һ�ữ���ų���CO32?��PO43?�����ӵĸ��ţ��ټ���AgNO3��Һ��������ɫ����������Һ��һ�����д�����Cl������ȷ��

��ϰ��ϵ�д�
�����Ŀ
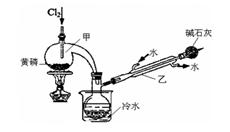
 2PCl3��2P+5Cl2
2PCl3��2P+5Cl2