��Ŀ����
ij������NH3��CO2�����ʵõ��ĸ�����ʯ���Ʊ����� ��NH4��2SO4���乤���������£�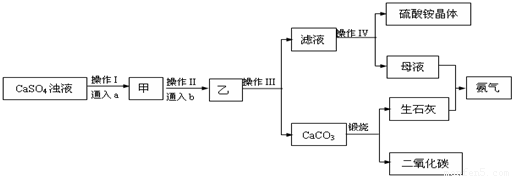
����������⣺
��1��д�����������Ʊ���NH4��2SO4 �Ļ�ѧ����ʽ______��
����I�Ͳ���II��ͨ���a ��b�����Ĺ�ϵ�ֱ�Ϊ______��
A��������CO2��������NH3 B��������NH3��������CO2
C��������CO2��������NH3 D��������NH3��������CO2
��2������III������Ϊ______��
��ʵ���ҽ��и������������IJ�������Ϊ______����A��H��ѡ��
A���Թ� B����ƿ C���ձ� D����Һ©��
E����ͨ©�� F��������ƿ G�������� H���ƾ���
��3������IV������IJ���������______��
A������ B������ C������ D����ȴ�ᾧ E������ F����Һ
�ò����õ���ĸҺ����������������Ҫ��______������鷽����______��
��4�����ʵ�ѭ��ʹ�ã��ܽ�Լ��Դ�����������л���ѭ��ʹ�õ�������______ ��д����ʽ��
���𰸡���������1�����ݲ��ַ�Ӧ���������������غ�ĽǶ���д��ѧ����ʽ��Ϊʹ��Һ�е�Ca2+��ȫ������Ӧ�ڼ��������·�Ӧ����CaCO3��
��2������IIIΪ�����Թ����Һ��ķ��룻
��3������IV����������
��4����Ͷ���ԭ�Ϻ�β���ɷ���ͬ����β����ѭ��ʹ�ã�
����⣺��1����NH3��CO2�����ʵõ��ĸ�����ʯ�ࣨCaSO4?2H2O���Ʊ����� ��NH4��2SO4������CO2����ˮ��NH3������ˮ��Ӧ��ͨ������NH3��ʹ��Һ�ʼ��ԣ�Ȼ����ͨ������CO2����Ӧ�Ļ�ѧ����ʽΪCaSO4+2NH3+CO2+H2O=��NH4��2SO4+CaCO3�����ʴ�Ϊ��CaSO4+2NH3+CO2+H2O=��NH4��2SO4+CaCO3����B��
��2������IIIΪCaCO3����Һ�ķ��룬ӦΪ���ˣ�����ʱ����Ҫ���������ձ�����ͨ©���Լ����������ʴ�Ϊ�����ˣ�CEG��
��3������IVΪ�����Թ������Һ�ķ��룬ӦΪ������������ȴ���������NH4��2SO4���壬Ȼ����ˣ�����NH4+ʱ��Ӧȡ��Һ��������NaOH�����ȣ������д̼�����ζ��������ʹʪ��ĺ�ɫʯ����ֽ������
�ʴ�Ϊ��BDE��NH4+��ȡ��Һ��������NaOH�����ȣ������д̼�����ζ��������ʹʪ��ĺ�ɫʯ����ֽ������
��4��aΪNH3��bΪCO2����Ͷ���ԭ�Ϻ�β���ɷ���ͬ����CO2��NH3��ѭ��ʹ�ùʴ�Ϊ��CO2��NH3���ʴ�Ϊ��CO2��NH3��
���������⿼���Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע��ѧϰ��Ҫ���ڻ��ۻ�����ѧʵ�����֪ʶ������ʵ��ԭ���ǽ�����Ĺؼ���
��2������IIIΪ�����Թ����Һ��ķ��룻
��3������IV����������
��4����Ͷ���ԭ�Ϻ�β���ɷ���ͬ����β����ѭ��ʹ�ã�
����⣺��1����NH3��CO2�����ʵõ��ĸ�����ʯ�ࣨCaSO4?2H2O���Ʊ����� ��NH4��2SO4������CO2����ˮ��NH3������ˮ��Ӧ��ͨ������NH3��ʹ��Һ�ʼ��ԣ�Ȼ����ͨ������CO2����Ӧ�Ļ�ѧ����ʽΪCaSO4+2NH3+CO2+H2O=��NH4��2SO4+CaCO3�����ʴ�Ϊ��CaSO4+2NH3+CO2+H2O=��NH4��2SO4+CaCO3����B��
��2������IIIΪCaCO3����Һ�ķ��룬ӦΪ���ˣ�����ʱ����Ҫ���������ձ�����ͨ©���Լ����������ʴ�Ϊ�����ˣ�CEG��
��3������IVΪ�����Թ������Һ�ķ��룬ӦΪ������������ȴ���������NH4��2SO4���壬Ȼ����ˣ�����NH4+ʱ��Ӧȡ��Һ��������NaOH�����ȣ������д̼�����ζ��������ʹʪ��ĺ�ɫʯ����ֽ������
�ʴ�Ϊ��BDE��NH4+��ȡ��Һ��������NaOH�����ȣ������д̼�����ζ��������ʹʪ��ĺ�ɫʯ����ֽ������
��4��aΪNH3��bΪCO2����Ͷ���ԭ�Ϻ�β���ɷ���ͬ����CO2��NH3��ѭ��ʹ�ùʴ�Ϊ��CO2��NH3���ʴ�Ϊ��CO2��NH3��
���������⿼���Ʊ�ʵ�鷽������ƣ���Ŀ�Ѷ��еȣ�ע��ѧϰ��Ҫ���ڻ��ۻ�����ѧʵ�����֪ʶ������ʵ��ԭ���ǽ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ