��Ŀ����
��������ʵ���ó��Ľ��ۻ�����Ľ�����ȷ���ǣ� ��
A���٢ݢ� B���ܢ� C���ۢܢޢ� D���ۢܢݢ�
| | ʵ����ʵ | ���ۻ���� |
| �� | ��40 g NaOH����1 L����ˮ�� | ����Һ�����ʵ���������Ϊ3��84%�������ʵ���Ũ��Ϊ1 mol��L-1 |
| �� | ��ʢ��1mL 0��1mol��L-1 AgNO3��Һ���Թ��еμ�0��1mol��L-1 NaCl��Һ���������г������ɣ��������еμ� 0��1mol��L-1 KI��Һ����ɫ����ת��Ϊ��ɫ������ | �����£�S��AgCl�� < S��AgI�� |
| �� | Mg��OH��2������ˮ��������NH4Cl��Һ | NH4Clˮ�������ԣ�Mg��OH��2��H+������Ӧ |
| �� | ij��NaX��ҺpH > 7 | ��HXΪ���� |
| �� | ��ij��Һ�м���2��KSCN��Һ����Һ���Ժ�ɫ��������Һ�м��뼸�����Ƶ���ˮ����Һ��Ϊ��ɫ | ˵����Һ��һ������Fe2+ |
| �� | ��ʢ��Ũ������Թ��зֱ����AlƬ��CuƬ��ǰ��û�����������߷�Ӧ���ң�������������ɫ���� | ��ԭ��Al < Cu |
| �� | BaSO4��ˮ��Һ�����Ժܲ� | BaSO4��������� |
B
�ٽ�40 g NaOH����1 L����ˮ�У�������֪����Һ�������Ҳ�����������ʵ���Ũ�ȣ����е�����ǡǡ˵��S��AgCl��> S��AgI����
��Mg��OH��2��Һ�д����ܽ�ƽ�⣺Mg��OH��2��s�� Mg2+��aq��+2OH����aq��,����NH4Cl��Һ��NH4+�ɽ��OH�����������ʹ�ܽ�ƽ�����ƣ�Mg��OH��2���ܽ�
Mg2+��aq��+2OH����aq��,����NH4Cl��Һ��NH4+�ɽ��OH�����������ʹ�ܽ�ƽ�����ƣ�Mg��OH��2���ܽ�
�����ڳ�������Ũ�����жۻ���
��BaSO4��ǿ����ʣ��������ܽ��̫С�����������ƶ������ӵ�Ũ�Ȳ���������ˮ��Һ�����Ժܲ�
��Mg��OH��2��Һ�д����ܽ�ƽ�⣺Mg��OH��2��s��
 Mg2+��aq��+2OH����aq��,����NH4Cl��Һ��NH4+�ɽ��OH�����������ʹ�ܽ�ƽ�����ƣ�Mg��OH��2���ܽ�
Mg2+��aq��+2OH����aq��,����NH4Cl��Һ��NH4+�ɽ��OH�����������ʹ�ܽ�ƽ�����ƣ�Mg��OH��2���ܽ������ڳ�������Ũ�����жۻ���
��BaSO4��ǿ����ʣ��������ܽ��̫С�����������ƶ������ӵ�Ũ�Ȳ���������ˮ��Һ�����Ժܲ�

��ϰ��ϵ�д�
�����Ŀ
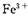 ˮ��ǿ��Fe2+������
ˮ��ǿ��Fe2+������