��Ŀ����
����Ŀ������Cu2O���ھ��������Ĵ����ܶ��ܵ���ע������Ϊ��ȡCu2O�����ַ���:
����a:��̿���ڸ��������»�ԭCuO
����b:��ⷨ����ӦΪ2Cu+H2O![]() Cu2O+H2��
Cu2O+H2��
(1)��֪:��2Cu(s)+![]() O2(g)=Cu2O(s)��H1=akJ/mol
O2(g)=Cu2O(s)��H1=akJ/mol
��C(s)+![]() O2(g)=CO(g) ��H2=bkJ/mol
O2(g)=CO(g) ��H2=bkJ/mol
��Cu(s)+![]() O2(g)=CuO(s)��H3=ckJ/mol
O2(g)=CuO(s)��H3=ckJ/mol
��a�з�Ӧ���Ȼ�ѧ����ʽ��:_________________��
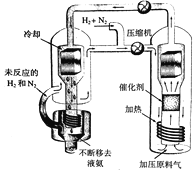
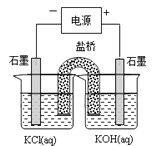
(2)����b������ȼ�ϵ��Ϊ��Դ��ͨ�����ӽ���Ĥ��ⷨ���Ƶ��Һ��OH-��Ũ�ȶ��Ʊ�����Cu2Oװ����ͼ��ʾ:

����ͼװ����D�缫Ӧ��______�缫��(�A����B��)
�ڸ����ӽ���ĤΪ______���ӽ���Ĥ(�����������)���õ��ص�B����ӦʽΪ:______��
��C����ӦʽΪ:__________��
(3)����ͬ����ĺ����ܱ������У������Ϸ����Ƶõ�����Cu2O�ֱ���д��ֽ�ˮ��ʵ��:
2H2O![]() 2H2(g)+O2(g) ��H>0��ˮ������Ũ����ʱ��t�仯�����ʾ:
2H2(g)+O2(g) ��H>0��ˮ������Ũ����ʱ��t�仯�����ʾ:

�����������ݷ���:
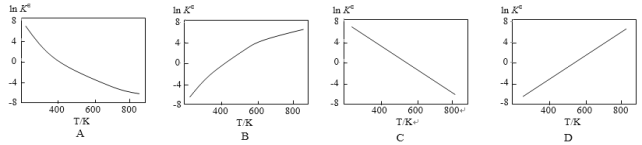
�ٴ�����Ч��:ʵ���_______ʵ���(�>����<��)��
��ͨ��ʵ��١��۷���,T1______T2(�>����<��)��
��ʵ��١��ڡ��۵Ļ�ѧƽ�ⳣ��K1��K2��K3�Ĵ�С��ϵΪ:_________��
���𰸡� 2CuO(s)+ C(s)= Cu2O(s)+ CO(g) ��H = (a+b-2c) kJmol-1 B �� 2Cu - 2e- + 2OH- =Cu2O + H2O N2H4 - 4e- + 4OH- =N2 ��+ 4 H2O �� �� K1=K2<K3
����������1�����ݸ�˹���ɽ���֪���Ȼ�ѧ����ʽ���μ���õ������Ȼ�ѧ����ʽ����2����ȼ�ϵ������ͨ����������ͨȼ�ϣ���CΪ������DΪ������ͭ�缫�������������ĵ缫����ʽΪ2Cu+2OH��-2e��=Cu2O+H2O����ͭΪ��������ԭ��صĸ�����������B��C��A��D�������ڸ����ܷ�Ӧ����������ӦΪ2Cu-2e��+2OH��=Cu2O+H2O���������ӽ���ĤӦ���������ӽ���Ĥ���ڵ��ص���������ʧ���ӵ�������Ӧ���۾�ԭ����и�������������Ӧ����д�缫��Ӧ����ʽ����3���ٴ���Ч��Խ�ߣ���Ӧ����Խ�죬����ƽ��ʱ��Խ�̣���ʵ��3�ﵽƽ������ʱ��̣���ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ��䣬ƽ�ⳣ�����䣬���¶����ߣ�ƽ�������ȷ����ƶ�����������Ӧ�����ƶ���ƽ�ⳣ�����ݴ˽��з�����
(1)���ݸ�˹���ɢ�+��-2���۵õ�2CuO(s)+ C(s)= Cu2O(s)+ CO(g) ��H = (a+b-2c) kJmol��1��(2)��ȼ�ϵ������ͨ����������ͨȼ�ϣ���CΪ������DΪ������ͭ�缫�������������ĵ缫����ʽΪ2Cu+2OH��-2e��=Cu2O+H2O����ͭΪ��������ԭ��ص�������������B��D��A��C����������ͼװ����D�缫Ӧ��B�缫���ڸ����ܷ�Ӧ����������ӦΪ2Cu-2e��+2OH��=Cu2O+H2O���������ӽ���ĤӦ���������ӽ���Ĥ���ڵ��ص���������ʧ���ӵ�������Ӧ���õ��ص�B����ӦʽΪ:2Cu - 2e- + 2OH- =Cu2O + H2O����ԭ����и�������������Ӧ����C����ӦʽΪ:N2H4 - 4e- + 4OH- =N2 ��+ 4 H2O��(3)��ʵ��٢���ȣ�ʵ��ڵ���ƽ��ʱ��̣���Ӧ����Խ�죬����Ч�ʸߣ��ʴ�Ϊ��������ʵ��3�ﵽƽ������ʱ��̣�ͨ��ʵ��١��۷���,T1<T2����ʵ��1��2�¶���ͬ��ƽ�ⳣ����ͬ����K1=K2���Ƚ�ʵ��2��3��ʵ��3�г�ʼˮ����Ũ����ʵ��2��һ��������Ӧ����ˮ����Ũ��С��ʵ��2��һ������ƽ��������Ӧ������У����¶����ߣ�ƽ�������ȷ����ƶ����÷�ӦΪ���ȷ�Ӧ������ƽ��������Ӧ�����ƶ���ƽ�ⳣ������K3��K2������K1=K2��K3��