��Ŀ����
11��ˮ��ʯ��IDHs����һ������ǽ������ϣ����������ϵ���ȼ���ȣ�ij����С���ͬѧͨ�����������Ʊ�þ��ˮ��ʯ��
��1��A��ҺΪһ��Ũ�ȵ�Mg��NO3��2��Al��NO3��2�Ļ����Һ������ʱ�����ձ����������⣬����Ҫ�IJ���������500mL����ƿ����ͷ�ιܣ�
��2��B��ҺΪc��NaOH��=1.6mol•L-1��c��Na2CO3��=0.8mol•L-1�Ļ����Һ��������ʱ�����ȡNaOH������Ϊ32g��B��Һ��c��Na+��=3.2mol•L-1��
��3������ʱ��������������������
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡ��ʹ�õ�������
��2������n=cV����NaOH��������c=$\frac{n}{V}$����B��Һ��c��Na+����
��3������ʱ��������������������
��� �⣺��1������500mL һ��Ũ�ȵ�Mg��NO3��2��Al��NO3��2�Ļ����Һ�IJ���Ϊ�����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ���Ҫʹ�õ������У�Կ�ס���ƽ���ձ���500mL����ƿ����ͷ�ιܡ��������ȣ����Ի�ȱ�ٵIJ�������Ϊ��500 mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��500 mL����ƿ����ͷ�ιܣ�
��2����ΪB��ҺΪc��NaOH��=1.6mol•L-1��c��Na2CO3��=0.8mol•L-1�Ļ����Һ����NaOH������m=40n=40cV=40��1.6��0.5=32g��c��Na+��=$\frac{n}{V}$=$\frac{1.6��0.5+0.8��0.5��2}{0.5}$=3.2mol•L-1���ʴ�Ϊ��32g��3.2��
��3������ʱ���������������������ʴ�Ϊ��������
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ���������㣬��ȷ����ԭ�������ǽ���ؼ���ע�������ʽ��Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ
1��NA���������ӵ�������ֵ�����и�����ȷ���ǣ�������
| A�� | 10mL��������Ϊ98%��H2SO4����ˮϡ����100 mL��H2SO4����������Ϊ9.8% | |
| B�� | ��NA��Na+��Na2O�ܽ���1Lˮ�У�Na+ �����ʵ���Ũ��Ϊ1mol•L-1 | |
| C�� | ��0.1mol�Ȼ�������1Lˮ�У�������Һ����0.1NA��Fe3+ | |
| D�� | �����£�1L 0.1mol•L-1��NH4NO3��Һ�е�ԭ����Ϊ0.2NA |
2��ʵ���е����в�����ȷ���ǣ�������
| A�� | ���Թ�ȡ���Լ�ƿ�е�Na2CO3��Һ������ȡ������Ϊ�˲��˷ѣ��ְѹ������Լ������Լ�ƿ�� | |
| B�� | Ba��NO3��2����ˮ���ɽ�����Ba��NO3��2�ķ�Һ����ˮ���У�����ˮ������ˮ�� | |
| C�� | ����������ʹNaCl ����Һ������ʱ��Ӧ����������NaCl ��Һȫ���������� | |
| D�� | �ƾ��Ʋ��������Ż�ʱ����ʪĨ������ |
19��������������ȷ���ǣ�������
| A�� | H2SO4��Ħ��������98 | |
| B�� | 2mol NO�к�2mol����2mol O | |
| C�� | ��������O2��O3��������ԭ�Ӹ�����ͬ | |
| D�� | 1mol O22-������Ϊ34g |
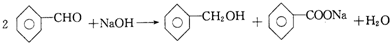
6�����෨��һ����֮��Ч�������еĿ�ѧ������ijͬѧ���±���ʾ��ʽ����ѧ֪ʶ���з��࣬���м� ���ǰ�����ϵ�����и����У��д��������ǣ�������
| ѡ�� | A | B | C | D |
| �� | ���� | ���������� | ����Ԫ�� | ��ѧ��Ӧ |
| �ҡ��� | ���������� | ���ӡ�ԭ�ӡ����� | ͭ��̼���� | ���Ϸ�Ӧ �ֽⷴӦ |
| A�� | A | B�� | B | C�� | C | D�� | D |
5��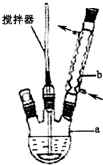 ����ȩ��Ũ����������ܷ����绯��Ӧ���ɱ��״��ͱ������Σ�ijʵ��С�����ø�ԭ���Ʊ������ı����ᣮ
����ȩ��Ũ����������ܷ����绯��Ӧ���ɱ��״��ͱ������Σ�ijʵ��С�����ø�ԭ���Ʊ������ı����ᣮ
����������ף������õ����й��������£�
��1���й����ʵ��ܶȡ��е㡢�ܽ���
��2��������Ϊ��ɫƬ״���壬�۵�122.4�棬��������ˮ�е��ܽ�ȼ��±�
ʵ�鷽������ͼʾ��Ӧװ���У����뷴Ӧ������¿���55�������ƿ�����ʳɺ�״����ɣ����װ�ã�������a�м�������ˮ������ʹ�����ܽ⣬Ȼ��Ӧ�����ת���Һ©���У���30ml��ȡ����3����ȡ������ȡ���ˮ���м���30g������ڽ�������������ŨHCl��ʪ�����ɫʯ����ֽ��죬��ˮԡ����ȴ�����������Ʒ����ش��������⣺
��1��д��װ��ͼ�в������������ƣ�a������ƿ��b�����Σ������ܣ�
��2��Ϊ�˽�һ���ᴿ�����ᣬͨ�������ؽᾧ�����У��ڽ��и�ʵ���������Ҫ���ȹ��ˣ����ֱ�����ȫ�ܺ�������Ҫ�ټ�����������ˮ��Ŀ����Ϊ�˼��ٳ��ȹ��˹�������ʧ�����ᣮ
��3����������IJ�Ʒ����ϴ�ӡ�������龭����������õ����Ƿ��Ǵ����ı�����ij��÷����Dzⶨ��ò�Ʒ���۵��Ƿ���122.4�森
��4�������������У�������ȩ��ȡ������Ϊ2.120g�����ջ�ȡ�Ĵ��������������Ϊ1.098g������IJ���Ϊ90.0%��
��5��ijͬѧ��Ϊ�ɴ���ȥ����ȡ�л����л�ȡ���״���Ϊ��ɸ�ʵ����Ҫ�IJ����������¶ȼơ�β�ӹܼ�������ƿ��ֱ�������ܡ���ƿ��
 ����ȩ��Ũ����������ܷ����绯��Ӧ���ɱ��״��ͱ������Σ�ijʵ��С�����ø�ԭ���Ʊ������ı����ᣮ
����ȩ��Ũ����������ܷ����绯��Ӧ���ɱ��״��ͱ������Σ�ijʵ��С�����ø�ԭ���Ʊ������ı����ᣮ
����������ף������õ����й��������£�
��1���й����ʵ��ܶȡ��е㡢�ܽ���
| ���� | ����Է��� ���� | �ܶ�/ �������ˮ�� | �����е�/�� | ���������ܽ��� |
| ����ȩ | 106 | 1.046 | 178.8 | ����ˮ���ɻ������Ҵ������ѡ������ȷ� |
| ���״� | 108 | 1.042 | 205.7 | ����ˮ���������Ҵ����ѡ����� |
| ������ | 122 | 1.271 | 249.2 | ����ˮ���������Ҵ������ѡ��ȷ¡���������̼�����Ȼ�̼ |
| ���������� | 144 | 1.442 | 249.3 | ����ˮ���Ҵ������͡������״����������� |
| �¶�/�� | 25 | 50 | 95 |
| �ܽ��/g | 0.17 | 0.95 | 6.8 |
��1��д��װ��ͼ�в������������ƣ�a������ƿ��b�����Σ������ܣ�
��2��Ϊ�˽�һ���ᴿ�����ᣬͨ�������ؽᾧ�����У��ڽ��и�ʵ���������Ҫ���ȹ��ˣ����ֱ�����ȫ�ܺ�������Ҫ�ټ�����������ˮ��Ŀ����Ϊ�˼��ٳ��ȹ��˹�������ʧ�����ᣮ
��3����������IJ�Ʒ����ϴ�ӡ�������龭����������õ����Ƿ��Ǵ����ı�����ij��÷����Dzⶨ��ò�Ʒ���۵��Ƿ���122.4�森
��4�������������У�������ȩ��ȡ������Ϊ2.120g�����ջ�ȡ�Ĵ��������������Ϊ1.098g������IJ���Ϊ90.0%��
��5��ijͬѧ��Ϊ�ɴ���ȥ����ȡ�л����л�ȡ���״���Ϊ��ɸ�ʵ����Ҫ�IJ����������¶ȼơ�β�ӹܼ�������ƿ��ֱ�������ܡ���ƿ��
10��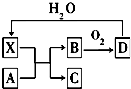 ��֪XΪһ�ֳ������Ũ��Һ����ʹ���Ƿ�ĩ��ڣ�A��X��Ӧ��ת����ϵ��ͼ��ʾ�����з�Ӧ���������ֲ��������ȥ���������й�˵����ȷ���ǣ�������
��֪XΪһ�ֳ������Ũ��Һ����ʹ���Ƿ�ĩ��ڣ�A��X��Ӧ��ת����ϵ��ͼ��ʾ�����з�Ӧ���������ֲ��������ȥ���������й�˵����ȷ���ǣ�������
 ��֪XΪһ�ֳ������Ũ��Һ����ʹ���Ƿ�ĩ��ڣ�A��X��Ӧ��ת����ϵ��ͼ��ʾ�����з�Ӧ���������ֲ��������ȥ���������й�˵����ȷ���ǣ�������
��֪XΪһ�ֳ������Ũ��Һ����ʹ���Ƿ�ĩ��ڣ�A��X��Ӧ��ת����ϵ��ͼ��ʾ�����з�Ӧ���������ֲ��������ȥ���������й�˵����ȷ���ǣ�������| A�� | ��ҵ�ϣ�Bת��ΪD�ķ�Ӧ����Ϊ���¡���ѹ��ʹ�ô��� | |
| B�� | ��AΪ����������A��X�������¼��ɷ�����Ӧ | |
| C�� | ��AΪ̼���ʣ�������Cͨ������NaClO��Һ�У�����ΪNaHCO3��HClO | |
| D�� | Xʹ���DZ�ڵ�������Ҫ������X��ǿ������ |
 2Fe3O4��S��+C��
2Fe3O4��S��+C��
 ��
��