��Ŀ����
��֪ij��Һ��ֻ����OH-��H+��NH
��Cl-�������ӣ�ijͬѧ�Ʋ�������Ũ�ȴ�С˳��������������ֹ�ϵ��
��c��Cl-����c��NH
����c��H+����c��OH-��
��c��NH
����c��OH-����c��Cl-����c��H+��
��c��NH
����c��Cl-����c��OH-����c��H+��
��c��Cl-����c��H+����c��NH
����c��OH-��
��д���пհף�
��1������Һ��ֻ�ܽ�һ�����ʣ����������
��2�����������ӵĹ�ϵ���Ϣۣ�������Ϊ
���������ӵĹ�ϵ���Ϣܣ�������Ϊ
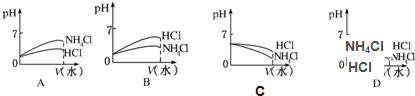
��3����pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı���������ͼ����ȷ���ǣ���ͼ����ţ�
��4��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc��HCl��
+ 4 |
��c��Cl-����c��NH
+ 4 |
��c��NH
+ 4 |
��c��NH
+ 4 |
��c��Cl-����c��H+����c��NH
+ 4 |
��д���пհף�
��1������Һ��ֻ�ܽ�һ�����ʣ����������
NH4Cl
NH4Cl
��������������Ũ�ȵĴ�С˳��Ϊ������ţ���
��
����2�����������ӵĹ�ϵ���Ϣۣ�������Ϊ
NH4Cl��NH3?H2O
NH4Cl��NH3?H2O
�����������ӵĹ�ϵ���Ϣܣ�������Ϊ
NH4Cl��HCl
NH4Cl��HCl
����3����pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı���������ͼ����ȷ���ǣ���ͼ����ţ�
B
B
��
��4��������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc��HCl��
��
��
c��NH3?H2O��������ڡ���С�ڡ����ڡ�����ͬ�������ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵΪc��H+������
����
c��OH-������������1���κ�ˮ��Һ�ж���OH-��H+��������ֻ��һ����ΪNH4Cl������笠�����ˮ��ʹ��Һ��������������
��2���ɢ������ӵĹ�ϵ��֪��Һ�Լ��ԣ���c��NH4+����c��Cl-��������ҺΪ�Ȼ�狀Ͱ�ˮ�Ļ����Һ���ɢ��е����ӹ�ϵ��֪��Һ�����ԣ���c��Cl-����c��H+����c��NH4+��������ҺΪ�������Ȼ�淋Ļ����Һ��
��3��pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı���������Ϊǿ����ʣ���pH�仯�̶ȴ�ϡ��ʱ�ٽ��Ȼ����笠�����ˮ�⣬��pH�仯С��
��4��������ϡ����Ͱ�ˮ��ϣ���Ũ����ȣ���ǡ����ȫ��Ӧ�����Ȼ�泥���Һ�����ԣ�����Һǡ�ó�����ʱӦΪ��ˮ���Ȼ�淋Ļ����Һ��������
��2���ɢ������ӵĹ�ϵ��֪��Һ�Լ��ԣ���c��NH4+����c��Cl-��������ҺΪ�Ȼ�狀Ͱ�ˮ�Ļ����Һ���ɢ��е����ӹ�ϵ��֪��Һ�����ԣ���c��Cl-����c��H+����c��NH4+��������ҺΪ�������Ȼ�淋Ļ����Һ��
��3��pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı���������Ϊǿ����ʣ���pH�仯�̶ȴ�ϡ��ʱ�ٽ��Ȼ����笠�����ˮ�⣬��pH�仯С��
��4��������ϡ����Ͱ�ˮ��ϣ���Ũ����ȣ���ǡ����ȫ��Ӧ�����Ȼ�泥���Һ�����ԣ�����Һǡ�ó�����ʱӦΪ��ˮ���Ȼ�淋Ļ����Һ��������
����⣺��1�����κ�ˮ��Һ�ж���OH-��H+��������ֻ��һ����ΪNH4Cl��笠�����ˮ�ⷽ��ʽΪNH4++H2O?NH3��H2O+H+����c��Cl-����c��NH4+����ˮ�������ԣ���c��H+����c��OH-������ˮ��ij̶Ⱥ�������c��Cl-����c��NH4+����c��H+����c��OH-�������ٷ��ϣ�
�ʴ�Ϊ��NH4Cl���٣�
��2���������ӵĹ�ϵ��֪��Һ�Լ��ԣ���c��NH4+����c��Cl-��������ҺΪ�Ȼ�狀Ͱ�ˮ�Ļ����Һ��������ΪNH4Cl��NH3��H2O��
���е����ӹ�ϵ��֪��Һ�����ԣ���c��Cl-����c��H+����c��NH4+��������ҺΪ�������Ȼ�淋Ļ����Һ��������ΪHCl��NH4Cl��
�ʴ�Ϊ��NH4Cl��NH3?H2O��NH4Cl��HCl��
��3��pH��ͬ��NH4Cl��Һ��HCl��Һϡ��ʱ�����ӵ�Ũ�ȶ���С����pH���������Ϊǿ����ʣ���pH�仯�̶ȴ��Ȼ��ˮ�������ԣ�ϡ�ʹٽ�ˮ�⣬������Ũ�ȼ�С�ı���С��������������Ũ�ȵı仯����pH�仯С��ֻ��B�������⣬
�ʴ�Ϊ��B��
��4���������Ũ����ͬ��ϡ����Ͱ�ˮ��ϣ���Һ�е�����Ϊ�Ȼ�泥���Һ�����ԣ�������Һǡ�ó����ԣ���ӦΪ��ˮ���Ȼ�淋Ļ����Һ����c��HCl����c��NH3?H2O������HClΪǿ����ʣ���ȫ���룬NH3��H2OΪ������ʣ�����ȫ���룬����ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵΪc��H+����c��OH-����
�ʴ�Ϊ��С�ڣ����ڣ�
�ʴ�Ϊ��NH4Cl���٣�
��2���������ӵĹ�ϵ��֪��Һ�Լ��ԣ���c��NH4+����c��Cl-��������ҺΪ�Ȼ�狀Ͱ�ˮ�Ļ����Һ��������ΪNH4Cl��NH3��H2O��
���е����ӹ�ϵ��֪��Һ�����ԣ���c��Cl-����c��H+����c��NH4+��������ҺΪ�������Ȼ�淋Ļ����Һ��������ΪHCl��NH4Cl��
�ʴ�Ϊ��NH4Cl��NH3?H2O��NH4Cl��HCl��
��3��pH��ͬ��NH4Cl��Һ��HCl��Һϡ��ʱ�����ӵ�Ũ�ȶ���С����pH���������Ϊǿ����ʣ���pH�仯�̶ȴ��Ȼ��ˮ�������ԣ�ϡ�ʹٽ�ˮ�⣬������Ũ�ȼ�С�ı���С��������������Ũ�ȵı仯����pH�仯С��ֻ��B�������⣬
�ʴ�Ϊ��B��
��4���������Ũ����ͬ��ϡ����Ͱ�ˮ��ϣ���Һ�е�����Ϊ�Ȼ�泥���Һ�����ԣ�������Һǡ�ó����ԣ���ӦΪ��ˮ���Ȼ�淋Ļ����Һ����c��HCl����c��NH3?H2O������HClΪǿ����ʣ���ȫ���룬NH3��H2OΪ������ʣ�����ȫ���룬����ǰ����c��H+���ͼ���c��OH-���Ĺ�ϵΪc��H+����c��OH-����
�ʴ�Ϊ��С�ڣ����ڣ�
���������⿼������Ũ�ȴ�С�ıȽϣ���Ŀ���ѣ�����ѧ���������ӵĹ�ϵ��������Һ�е����ʣ���ȷ��Һ�еĵ����ˮ���ǽ����Ĺؼ�����ע�⣨3������4����ѧ�������ѵ㣬��ǿ�ᡢ�����ϡ�����ƣ�

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
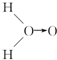 ��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
 �ķе����
�ķе����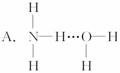

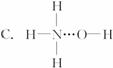

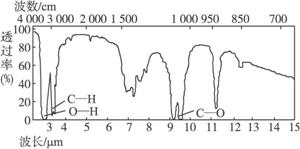
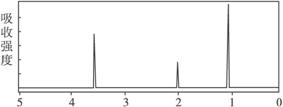
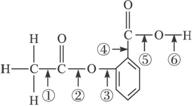
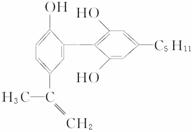


 �ӷ�Ӧʽ�� ��
�ӷ�Ӧʽ�� ��