��Ŀ����
����ֲ���纣������β��Ⱥ��зḻ�ĵ�Ԫ�أ�ʵ���ҴӺ�������ȡ����������£�
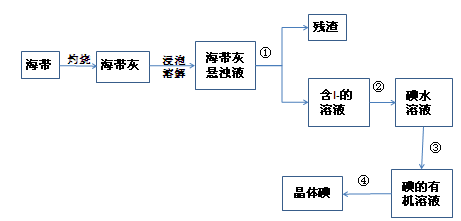
��1����ȡ��Ĺ������й�ʵ��IJ������Ƣ� �� �� ��
��2��д�����̢����йط�Ӧ�����ӷ���ʽ ��
�÷�Ӧ���������� ������������ ��
��3����ȡ��Ĺ����У���ѡ����л��Լ��� ��
�ڵ�ˮ�м��������л��Լ������ã��ɹ۲쵽��������

��1����ȡ��Ĺ������й�ʵ��IJ������Ƣ� �� �� ��
��2��д�����̢����йط�Ӧ�����ӷ���ʽ ��
�÷�Ӧ���������� ������������ ��
��3����ȡ��Ĺ����У���ѡ����л��Լ��� ��
| A���ƾ� | B��ˮ | C�����Ȼ�̼ | D������ |
��1���ٹ��� ������ ����ȡ��Һ ������
��2��Cl2 + 2I- 2Cl- + I2�� Cl2��I2
2Cl- + I2�� Cl2��I2
��3��C���ֲ㣬�ϲ���ɫ��dz������Ϊ��ɫ���²�Ϊ�Ϻ�ɫ
��2��Cl2 + 2I-
 2Cl- + I2�� Cl2��I2
2Cl- + I2�� Cl2��I2��3��C���ֲ㣬�ϲ���ɫ��dz������Ϊ��ɫ���²�Ϊ�Ϻ�ɫ
�����������1�������ҵ�����Һ��Ҫͨ�����˵ķ���ʵ�ַ��롣�����ӱ�Ϊ���ʵ⣬���ϼ����ߣ�ʧȥ���ӣ������������ڵ��ʵ��������л��ܼ��У����Դӵ�ˮ��������ʵ���Ҫ��ȡ��Ҫ�ӵ���л��ܼ��з�������ʵ⣬��Ҫͨ������ķ���ʵ�֡�
��2����Ԫ�صķǽ�����ǿ�ڵ�Ԫ�أ��������ܰѵ������������ɵ��ʵ⣬�����̢����йط�Ӧ�����ӷ���ʽ��Cl2+2I����I2+2Cl�������������������������������ǵ��ʵ⡣
��3����ȡ�ʺ��������ڲ�ͬ�ܼ��е��ܽ��Բ�ͬ�������һ�ַ�����ѡ�õ���ȡ����ԭ���ǣ��ٺ�ԭ��Һ�е��ܼ��������ܸ����ܷ�����ѧ��Ӧ���������ڸ��ܼ����ܽ��ҪԶ����ԭ�ܼ����ƾ���������ˮ�ǻ��ܵģ�������Ϊ��ȡ�������Դ�ѡC���������Ȼ�̼���ܶȴ��ڿ����ģ���ʵ���������ϲ���ɫ��dz������Ϊ��ɫ���²�Ϊ�Ϻ�ɫ��
�����������Ǹ߿��еij������ͣ����ڻ�����ʵ����Ŀ��飬������۽̲Ļ���֪ʶ�������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԣ�����������ѧ���淶�Ͻ���ʵ������������Լ��淶�Ķ��ֲ�������������������ѧ����ѧ��������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
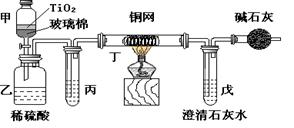
 ��Һ��������ú�Һ
��Һ��������ú�Һ ��Һ��������ú�Һ
��Һ��������ú�Һ