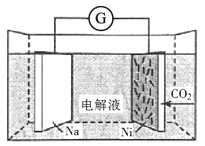
��Ŀ����
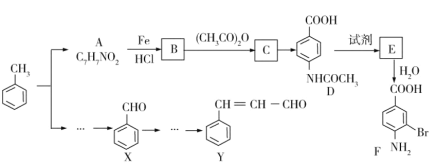
����Ŀ��������Ҫ���л������ϳ��м���F��Y���üױ�Ϊ��Ҫԭ�ϲ���·���Ƶã�
��֪����![]()
![]()
![]()
��2CH3CHO![]() CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO![]() CH3CH=CHCHO
CH3CH=CHCHO
��![]() ���¡���ѹ������NaOH����ȡ����Ӧ
���¡���ѹ������NaOH����ȡ����Ӧ
��ش��������⣺
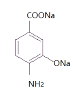
(1)д��X�����ƣ�__________________��
(2)д��![]() ��A�Ļ�ѧ����ʽ��_______________________________________��
��A�Ļ�ѧ����ʽ��_______________________________________��
(3)D��E���Լ�Ϊ��______________________��
(4)д��1molF�ڸ��¡���ѹ����NaOH�ķ�Ӧ��____________________________________��
(5)д��������������A��ͬ���칹���һ�ֽṹ��ʽ��________________��
�ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�Ӣ��ܷ���������Ӧ
(6)��������������Ϣ��������л���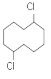 �ϳ�
�ϳ�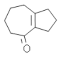 ����ͼ�������Լ���ѡ��_______________
����ͼ�������Լ���ѡ��_______________
���𰸡�����ȩ ![]() +HNO3(Ũ��
+HNO3(Ũ��
![]() +H2O Һ�塢FeBr3
+H2O Һ�塢FeBr3 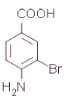 +3NaOH
+3NaOH
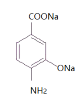 +NaBr+2H2O
+NaBr+2H2O ![]() ��
��![]() ��
��![]() (��дһ�֣�
(��дһ�֣� 
��������
�������Ʒ�����D�Ľṹ��ʽ�����Ƴ�CΪ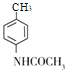 ��BΪ
��BΪ![]() ��AΪ
��AΪ![]() ��EΪ
��EΪ ���ݴ˽��
���ݴ˽��
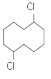
(1)XΪ![]() �������DZ���ȩ����Ϊ������ȩ��
�������DZ���ȩ����Ϊ������ȩ��
(2) ![]() ����ᷴӦ����
����ᷴӦ����![]() ��ˮ����ѧ����ʽΪ
��ˮ����ѧ����ʽΪ![]() +HNO3(Ũ)
+HNO3(Ũ)![]()
![]() +H2O������
+H2O����Ϊ��![]() +HNO3(Ũ)
+HNO3(Ũ)![]()
![]() +H2O��
+H2O��
(3)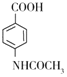 ��
�� ������Br2�ڴ��������·���ȡ����Ӧ���Լ�Ϊ��Һ�塢FeBr3����Ϊ��Һ�塢FeBr3��
������Br2�ڴ��������·���ȡ����Ӧ���Լ�Ϊ��Һ�塢FeBr3����Ϊ��Һ�塢FeBr3��
(4) ![]() �ڸ��¡���ѹ����NaOH�ķ�Ӧ��������Ϣ��-Br��ת��Ϊ-ONa������-COOHת��Ϊ-COONa����Ӧ�ķ���ʽΪ
�ڸ��¡���ѹ����NaOH�ķ�Ӧ��������Ϣ��-Br��ת��Ϊ-ONa������-COOHת��Ϊ-COONa����Ӧ�ķ���ʽΪ![]() +3NaOH
+3NaOH
 +NaBr+2H2O������
+NaBr+2H2O������![]() +3NaOH
+3NaOH
 +NaBr+2H2O��
+NaBr+2H2O��
(5)AΪ![]() �����������ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ����ܷ���������Ӧ
�����������ٱ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ����ܷ���������Ӧ
��ͬ���칹�壬����ȡ����Ӧλ�ڶ�λ����1��ȡ��������ȩ��������Ϊ-CHO��-OOCH��-NHCHO������һȡ����Ϊ-NHOH��-NH2��-OH���ṹ��ʽΪ![]() ��
��![]() ��
��![]() (��дһ�֣�����Ϊ��
(��дһ�֣�����Ϊ��![]() ��
��![]() ��
��![]() (��дһ�֣���
(��дһ�֣���
(6)�� �ϳ�
�ϳ�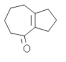 ����������Ϣ2CH3CHO
����������Ϣ2CH3CHO![]() CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO![]() CH3CH=CHCHO������
CH3CH=CHCHO������ ��ˮ�⣬��-Clת��Ϊ-OH����������ͪ�����������Ϣ�õ�����ϳ�·��Ϊ
��ˮ�⣬��-Clת��Ϊ-OH����������ͪ�����������Ϣ�õ�����ϳ�·��Ϊ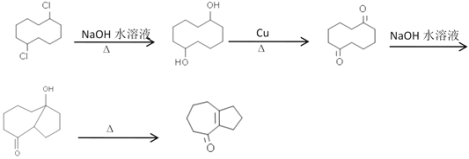 ����Ϊ��
������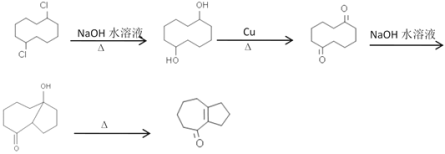 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�