��Ŀ����
12���Ȼ���ͭ��CuCl���ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ����������ɫ��ʽ�Σ������Ե�Ʒ�Һ����Ҫ��Cu2+��Fe3+�����Ʊ��Ȼ���ͭ�Ĺ�������ͼ��ͼ�ף��������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ�ң�
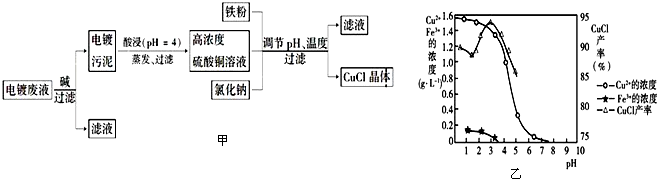
��֪����������Ũ��Ϊ1mol•L-1ʱ��Fe��OH��3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu��OH��2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
��1�����ʱ������Ӧ�����ӷ���ʽ��Cu��OH��2+2H+=Cu2++2H2O������CuCl����ʱ�����pH��3���ң�
��2�����ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ2Cu2++2Cl-+Fe=2CuCl��+Fe2+
��3��������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ���Ǽӿ��Ҵ���ˮ����������ֹCuCl������������
��4����Ʒ�˳�ʱ������Һ����Ҫ�ֳ���Na2SO4��FeSO4���������Һ�л�ȡFeSO4•7H2O���壬����Ҫ֪���IJ�ͬ�¶��������ƺ������������ܽ�ȣ�
��5���������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��2CuSO4+Na2SO3+2NaCl+H2O=2CuCl��+2Na2SO4+H2SO4��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����OH-�к��˷�Ӧ�е�H+��������ƽ�������ƶ������CuCl�IJ��ʣ�����OH-Ũ�ȹ���ʱ��Cu+����OH-��ϣ�������������ͭ���Ӷ�������CuCl�IJ��ʣ�
���� ��Ʒ�Һ�м���������Cu��OH��2��Fe��OH��3��������pHԼΪ4ʱ��������õ�����ͭ��Һ����������������������ͭ��Һ�м����Ȼ��ơ���������������ԭ��Ӧ����CuCl������2Cu2++2Cl-+Fe=2CuCl��+Fe2+�����������ҺΪ�������������CuCl�����ʺ���ĿҪ������⣮
��1����pHԼΪ4ʱ��������õ�����ͭ��Һ����������������˵��������ͭ�����ܽ⣬��ͼ2��֪������CuCl���������pHӦΪCuCl�IJ�������������������ʽ��٣�ӦԼΪ3���ң�
��2��������ͭ��Һ�м����Ȼ��ơ���������������ԭ��Ӧ����CuCl������2Cu2++2Cl-+Fe=2CuCl��+Fe2+���ʴ�Ϊ��2Cu2++2Cl-+Fe=2CuCl��+Fe2+��
��3���������Ϣ��֪CuCl����ˮ���������Ҵ����ڿ����лᱻѸ��������Ϊ��ֹ����Ӧ�����������ڸ����ջ����пɼӿ��Ҵ���ˮ��������
��4����Ʒ�˳�ʱ������Һ����Ҫ�ֳ���Na2SO4��FeSO4����ȡFeSO4•7H2O���壬����Ҫ֪�����������ƺ����������ܽ�����¶ȵı仯�������
��5���������ۻ�����������������������Ϊ��ԭ����ʧ���ӣ���Ӧ����ʽΪ��2 CuSO4+Na2SO3+2NaCl+H2O=2CuCl��+2 Na2SO4+H2SO4������ϡ����Һ���������ӣ���ƽ�������ƶ����������Ȼ���ͭ�IJ�������������������Ũ�ȴ�һ���̶�ʱ�Դﵽ������ͭ���ܶȻ��������
��� �⣺��1����pHԼΪ4ʱ��������õ�����ͭ��Һ����������������˵��������ͭ�����ܽ⣬��Ӧ�����ӷ���ʽΪCu��OH��2+2H+=Cu2++2H2O����ͼ2��֪������CuCl���������pHӦΪCuCl�IJ�������������������ʽ��٣�ӦԼΪ3���ң�
�ʴ�Ϊ��Cu��OH��2+2H+=Cu2++2H2O��3��
��2��������ͭ��Һ�м����Ȼ��ơ���������������ԭ��Ӧ����CuCl������2Cu2++2Cl-+Fe=2CuCl��+Fe2+��
�ʴ�Ϊ��2Cu2++2Cl-+Fe=2CuCl��+Fe2+��
��3���������Ϣ��֪CuCl����ˮ���������Ҵ����ڿ����лᱻѸ��������Ϊ��ֹ����Ӧ�����������ڸ����ջ����пɼӿ��Ҵ���ˮ��������
�ʴ�Ϊ���ӿ��Ҵ���ˮ����������ֹCuCl������������
��4����Ʒ�˳�ʱ������Һ����Ҫ�ֳ���Na2SO4��FeSO4����ȡFeSO4•7H2O���壬����Ҫ֪�����������ƺ����������ܽ�����¶ȵı仯�������
�ʴ�Ϊ��Na2SO4��FeSO4����ͬ�¶��������ƺ������������ܽ�ȣ�
��5������������Ϊ��ԭ����ʧ���ӣ�����������ԭ��Ӧ����Ӧ����ʽΪ��2 CuSO4+Na2SO3+2NaCl+H2O=2CuCl��+2 Na2SO4+H2SO4������ϡ����Һ���������ӣ���ƽ�������ƶ����������Ȼ���ͭ�IJ�������������������Ũ�ȴ�һ���̶�ʱ�Դﵽ������ͭ���ܶȻ�������������������ͭ�����Ե���pH��3.5��
�ʴ�Ϊ��2CuSO4+Na2SO3+2NaCl+H2O=2CuCl��+2 Na2SO4+H2SO4��OH-�к��˷�Ӧ�е�H+��������ƽ�������ƶ������CuCl�IJ��ʣ�����OH-Ũ�ȹ���ʱ��Cu+����OH-��ϣ�������������ͭ���Ӷ�������CuCl�IJ��ʣ�
���� ���⿼���Ʊ���������ƣ�������ѧ���ķ���������ʵ�������Ŀ��飬ע����������Ϣ������ʱע�����⣬������ѧ�������������Ѷ��еȣ�

 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�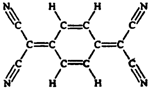 Ŀǰ���������Ѻϳ��˼������л������壬TCNQ������֮һ��TCNQ�ķ��ӽṹ��ͼ��ʾ�����й���TCNQ˵��������ǣ�������
Ŀǰ���������Ѻϳ��˼������л������壬TCNQ������֮һ��TCNQ�ķ��ӽṹ��ͼ��ʾ�����й���TCNQ˵��������ǣ�������| A�� | �����ʻ�ѧ���ʻ��ã��������ӳ� | |
| B�� | �÷��Ӳ���ƽ����ӣ�����������ԭ�ӹ��� | |
| C�� | ����ʽΪC12H4N4 | |
| D�� | ������������ˮ |
| A�� | H2O2��Һ������KMnO4��Һ��Ӧ��2MnO4-+3H2O2+6H+�T2Mn2++6H2O+4O2�� | |
| B�� | ��Fe��NO3��3��Һ�м��������HI��Һ��2NO2-+8H++6I-�T3I2+2NO��+4H2O | |
| C�� | ����SO2ͨ��̼������Һ�У�CO32-+SO2�TCO2+SO32- | |
| D�� | 0.01 mol•L-1 NH4Al��SO4��2��Һ��0.02 mol•L-1 Ba��OH��2��Һ�������ϣ�NH4++Al3++2SO32-+2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3•H2O |
| ����ʽ | ��� | �ȷֽ��¶� | �۵� | ˮ���ԣ�20�棩 |
| CO��NH2��2•H2O2 | ��ɫ���� | 45�� | 75-85�� | 500g•L-1 |
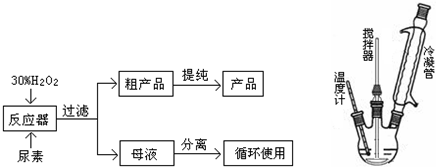
��ش��������⣺
��1��д���ϳɹ��������صĻ�ѧ����ʽ��CO��NH2��2+H2O2=CO��NH2��2•H2O2��
��2����Ӧ��������������ˮ���£���ϡ����¡��������룻��Ӧ���ļ��ȷ�ʽ�Ǣڣ�
��ֱ�Ӽ��ȣ���ˮԡ���ȣ�������ԡ���ȣ��ܱ�ԡ
��3��������ѡ�õIJ����Dz������������ʻ����ʲ��ϵ�ԭ���������ױ����������⣩��ʴ��
��4����ĸҺ�з����H2O2�����أ����õ��Ǽ�ѹ��������ȴ�ᾧ�ķ�������ԭ���ǽ��������¶ȣ���ֹ��������ֽ⣮
��5�������������ĸߵ�ֱ�Ӿ�����Ʒ���������ϸ��Ʒ�л������ĺ�����16%���൱�����к�H2O234%����Ϊ��ȷ�����ò�Ʒ�ϸ�����ʼ�Ա��ȡ������Ʒ2.000g���ܽ���ˮ����250mL����ƿ�ж��ݣ�ȷ��ȡ����25.00mL��Һ����ƿ�У�����1mL 6mol/LH2SO4��Ȼ����0.1000mol/L KMnO4����Һ�ζ���Ʒ�е�H2O2��KMnO4��Һ�������ط�Ӧ�������������һ��ʱ����Һ��dz��ɫ�Ұ�����ڲ���ɫ�����εζ�ƽ������KMnO4��Һ6.000mL��
����ɲ���ƽ���л�ѧ����ʽ��
2MnO4-+5H2O2+6H+=2Mn2++8H2O+5O2
��KMnO4��ҺӦʢ������ʽ�ζ����У����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õĻ���������ƫ�ߣ��ƫ�ߡ�����ƫ�͡����䡱����
�۸��ݵζ����ȷ����Ʒ�������ϸ���ϸ��ϸ�������������������Ϊ12%����
| A�� | 10mL O2��10mL CO2������ͬ�ķ����� | |
| B�� | 0.5 mol H2O��0.5 mol CO������ͬ�ķ����� | |
| C�� | ͬ��ͬѹ�£�10mL N2��10mL NO������ͬ��ԭ���� | |
| D�� | 1 mol Fe��1 mol Cu�ڳ�����������ԭ������ͬ���������ͬ |
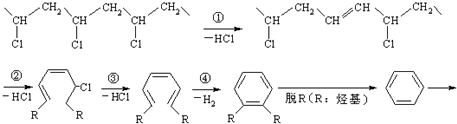
 ��CO������Ϊ���к���������HCl��
��CO������Ϊ���к���������HCl��