��Ŀ����
��ijNaOH��Һ��ͨ��CO2�����õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ������M����μ������ᣬ�������������V��CO2���������������V(HCl)�Ĺ�ϵ����ͼ��ʾ�������з������жϲ���ȷ���ǣ�����CO2���ܽ⣩ �� ��
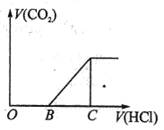

| A����OB=0�����γ���ҺM�����������ӷ�Ӧ����ʽΪ��OH-+CO2=HCO3- |
| B����OB=BC������ҺMΪNa2CO3��Һ |
| C����OB��BC������ҺM�д������ڵ�������ΪCO32-��HCO3- |
| D����3OB=BC������ҺM��c��NaHCO3��=2c��Na2CO3�� |
C
��Na2CO3��Һ����μ������ᣬ��Ӧԭ��Ϊ��
Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+CO2+H2O������Na2CO3��NaHCO3���������������ȡ���A����OA=0��������ʼ�μ���������������ɣ���M�е�����NaHCO3����ӦΪCO2+OH��=HCO3������B����OB=BC��˵������HCO3�����ĵ�H+��HCO3������CO2���ĵ�H+��ȣ�����е�����ΪNa2CO3����C��OB��BC��˵��������CO2����H+���ڲ���CO2���ĵ�H+������������ӦΪNaOH��Na2CO3����OB��BC��˵��������CO2����H+С�ڲ���CO2���ĵ�H+����M������ӦΪNa2CO3��NaHCO3����D��3OB=BC����������ΪNa2CO3��NaHCO3����ΪNa2CO3��NaHCO3����c��NaHCO3=2c(Na2CO3)��
Na2CO3+HCl=NaCl+NaHCO3��NaHCO3+HCl=NaCl+CO2+H2O������Na2CO3��NaHCO3���������������ȡ���A����OA=0��������ʼ�μ���������������ɣ���M�е�����NaHCO3����ӦΪCO2+OH��=HCO3������B����OB=BC��˵������HCO3�����ĵ�H+��HCO3������CO2���ĵ�H+��ȣ�����е�����ΪNa2CO3����C��OB��BC��˵��������CO2����H+���ڲ���CO2���ĵ�H+������������ӦΪNaOH��Na2CO3����OB��BC��˵��������CO2����H+С�ڲ���CO2���ĵ�H+����M������ӦΪNa2CO3��NaHCO3����D��3OB=BC����������ΪNa2CO3��NaHCO3����ΪNa2CO3��NaHCO3����c��NaHCO3=2c(Na2CO3)��

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
 B
B D
D ����
����