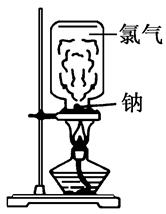
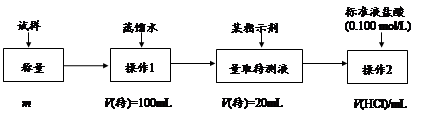��Ŀ����
�ҹ�����ר�Һ�°����ڴ��£��Ľ��������ˡ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�������������⣺
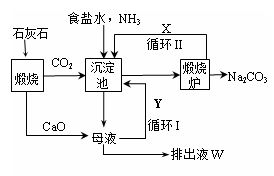
��1���������Ƽ���Ƶõġ���� (�ѧʽ)��
��2������������Ƽ��������Ҫ�Ĺ�ҵ�Ƽ�����б����У�����ȷ���� ��
ijʵ��С�飬��������װ��ģ�⡰�����Ƽ����
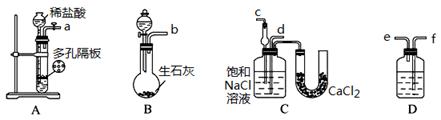
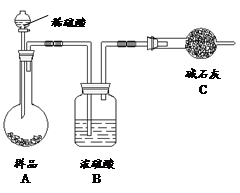
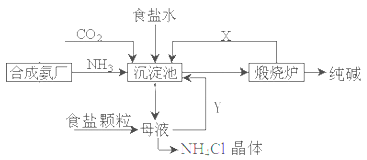
��3��ȡ������������װ�ã�˳��Ϊ��(a)��( )��( )��( )��(b)��( )��
���������Ժ�װ��ҩƷ��Ӧ������ װ�ã���������ĸ���ȷ�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������塣
��4��C�������θ���ܶ�����ֱ���ܣ��������� ��D��Ӧѡ�õ�Һ��Ϊ ��
��5��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ�� (ע����ı���ʽ�����õ��йط��ŵĺ���)��
��1���������Ƽ���Ƶõġ���� (�ѧʽ)��
��2������������Ƽ��������Ҫ�Ĺ�ҵ�Ƽ�����б����У�����ȷ���� ��
| | | ��� | �����Ƽ |
| A | ԭ�� | ʳ�Ρ���������ʯ�� | ʳ�Ρ�������������̼ |
| B | ���ܵĸ����� | �Ȼ��� | �Ȼ�� |
| C | ѭ������ | ������������̼ | �Ȼ��� |
| D | ���� | ԭ���ã��豸���ӣ��ܺĸ� | ԭ�������ʸߣ��������� |
ijʵ��С�飬��������װ��ģ�⡰�����Ƽ����

��3��ȡ������������װ�ã�˳��Ϊ��(a)��( )��( )��( )��(b)��( )��
���������Ժ�װ��ҩƷ��Ӧ������ װ�ã���������ĸ���ȷ�����Ӧ��ֱ�����������岻������C���ܽ�ʱ����ͨ����һװ���в��������塣
��4��C�������θ���ܶ�����ֱ���ܣ��������� ��D��Ӧѡ�õ�Һ��Ϊ ��
��5��C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪ ��
��6����Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ����������ɱ�ʾΪ�� (ע����ı���ʽ�����õ��йط��ŵĺ���)��
��1��Na2CO3
��2��A.C
��3��f e d c ��2�֣� B
��4�������� ����NaHCO3��Һ
��5��CO2+NH3+NaCl+H2O��NaHCO3��+NH4Cl
��6��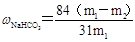 �� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ��������
�� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ��������
��2��A.C
��3��f e d c ��2�֣� B
��4�������� ����NaHCO3��Һ
��5��CO2+NH3+NaCl+H2O��NaHCO3��+NH4Cl
��6��
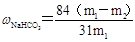 �� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ��������
�� m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ�������������������1���������Ƽ���Ƶõġ����̼���ƣ���ѧʽΪNa2CO3��
��2��A�����ԭ���У�ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼���������������Ƽԭ���У�ʳ�Ρ�������������̼����A����B��������ܵĸ�����Ϊ�Ȼ��ƣ������Ƽ���ܵĸ������Ȼ�泥���B��ȷ��C�����ѭ�����ʣ�������������̼�������Ƽѭ�����ʣ��Ȼ��ƣ�������̼����C����D�����ԭ�ϣ�ʳ�κ�ʯ��ʯ�����ˣ���Ʒ����Ĵ��ȸߣ�����Ʒ���Ͷ�����̼�����Ի���ѭ��ʹ�ã����첽����ʺ��ڴ��ģ���������豸���ӣ��ܺĸߣ���������ȱ�㻹����ԭ��ʳ�ε�������ֻ��72%��74%�������Ƽ�����ŵ���ʹʳ�ε���������ߵ�96%���ϣ��������٣���D��ȷ����ѡAC��
��3��Aװ�����Ʊ�CO2��Bװ�����Ʊ�NH3�����ڰ�����������ˮ��Ҫ�ø���ܷ�ֹ����������CO2�е��Ȼ������廹��Ҫ��ȥ�������ȷ������˳��Ϊ(a)��(f)��(e)��(d)��(b)��(c)������CO2��ˮ�е��ܽ��С�����Ҫ���Ȳ���������Ȼ����ͨ��CO2���壬����Ӧ������Bװ���ȷ�����Ӧ��
��4��������������ˮ�����C�������θ���ܶ�����ֱ���ܵ������Ƿ���������ȥCO2�е��Ȼ�������Ӧ���ñ���NaHCO3��Һ����D��Ӧѡ�õ�Һ��Ϊ����NaHCO3��Һ��
��5��Cװ�����Ʊ�̼�����Ƶģ�����C�й��ƿ�ڲ���������ܻ�ѧ����ʽΪCO2+NH3+NaCl+H2O��NaHCO3��+NH4Cl��
��6����m1Ϊ��Ʒ������m2Ϊ���Ⱥ��Ʒ�������������̼�����Ʒֽ�ķ���ʽ��֪
2NaHCO3
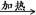 Na2CO3��H2O��CO2�� �����������١�m
Na2CO3��H2O��CO2�� �����������١�m168g 106g 62g
X m1��m2
���X��

���Դ�����̼�����Ƶ����������ɱ�ʾΪ
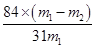 ��
��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

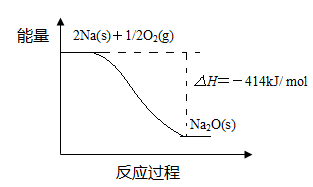
 Ni + 2NaCl����������Ӧʽ��_____��
Ni + 2NaCl����������Ӧʽ��_____��