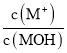��Ŀ����
����Ŀ�������ʼ������������ǵ����������������ء�
(1)Fe3+�Ļ�̬����۵����Ų�ʽΪ_____��Ӧ��ԭ�ӽṹ�Ƚ� Fe ��ͬ���ڵ� Mn ����������(I3)�Ĵ�С��I3(Mn)______I3(Fe)(���������)��������______��
(2)Fe��Fe2+��Fe3+������ CO��SCN-��CN-��H2NCONH2(����)�ȶ��������γɺܶ������
������� Fe(CO)5 ���۵�-20�棬�е� 103�棬�������Ʊ�������Fe(CO)5 �Ľṹ��ͼ��ʾ�����й��� Fe(CO)5 ˵������ȷ����____��
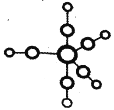
A.Fe(CO)5 �Ƿ��Ӿ���
B.Fe(CO)5 �� Fe ԭ�ӵ�������C22-��Ϊ�ȵ�����
C.Fe(CO)5 �� �� ���� �� ��֮��Ϊ 1:1
D.Fe(CO)5=Fe+5CO ��Ӧ��û���»�ѧ������
��CN-�ĵ���ʽΪ_____��
��H2NCONH2(����)�� N��C ԭ�ӵ��ӻ���ʽ�ֱ�Ϊ_____��______��������ص� 4 ��Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_____��1 mol H2NCONH2(����) �����к��� �� ������ĿΪ_____��
(3)NaCl ��MgO ���������ӻ����NaCl ���۵�Ϊ 801.3 �棬MgO ���۵�ߴ� 2800�档������־����۵������Ҫԭ����_____��
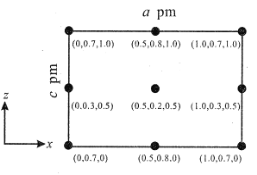
(4)�Ծ�������Ϊ��λ���Ƚ���������ϵ���Ա�ʾ�����и�ԭ�ӵ�λ�ã�����ԭ�ӷ������ꡣCsSiB3O7 ��������ϵ(��������)����������Ϊ a pm��b pm��c pm����ͼΪ�� y ��ͶӰ�ľ��������� Cs ԭ�ӵķֲ�ͼ��ԭ�ӷ������ꡣ�ݴ��ƶϸþ�����Cs ԭ�ӵ���ĿΪ_____��
���𰸡�3d5 �� Mn ԭ��ʧȥ 2 �����Ӻ���۵����Ų�ʽΪ 3d5�����ڰ����״̬�����ȶ� D ![]() sp3 sp2 N��O��H��C 7NA ����þ�������������ӵİ뾶��С��������������ܸ��� 4
sp3 sp2 N��O��H��C 7NA ����þ�������������ӵİ뾶��С��������������ܸ��� 4
��������
��1��Fe��26��Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��ʧȥ3�������γ�Fe3��������Fe3���Ļ�̬����۵����Ų�ʽΪ��3d5����Mn2��ת��ΪMn3��ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬תΪ���ȶ���3d4״̬��Ҫ�������϶࣬��Fe2��ת��ΪFe3��ʱ��3d�ܼ��ɲ��ȶ���3d6���ȶ���3d5�����״̬����Ҫ���������Ҫ�٣�����I3��Mn����I3��Fe����
�ʴ�Ϊ��3d5������Mn ԭ��ʧȥ 2 �����Ӻ���۵����Ų�ʽΪ 3d5�����ڰ����״̬�����ȶ���
��2����A�������Fe(CO)5���۷е�ܵͣ����ڷ��Ӿ��壬��A��ȷ��
B��Fe(CO)5��Feԭ�ӵ�����ΪCO����C22��ԭ��������ȣ��۵�����Ҳ��ͬ��������C22����Ϊ�ȵ����壬��B��ȷ��
C��Fe��CO�γ�5����λ����������ÿ��CO�����к���1��������2������������Fe(CO)5������������֮��Ϊ1��1����C��ȷ��
D����Ӧ�õ�Fe���ʣ��γɽ���������D����
��ѡ��D��
��CN����N2��Ϊ�ȵ����壬����N2��д�����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��H2NCONH2(����)��N��C ԭ�ӵļ۲���ӶԸ����ֱ���4��3�����ݼ۲���ӶԻ��������ж�N��Cԭ�ӵ��ӻ���ʽ�ֱ�Ϊsp3��sp2��ͬһ����Ԫ�أ����һ����������ԭ��������������������ƣ�����IIA�塢��VA���һ�����ܴ���������Ԫ�أ��⼸��Ԫ�ص�һ�����ܴ�С˳����N��O��C��H��ÿ��H2NCONH2�����к���7������������1 mol H2NCONH2�����أ������к�����������ĿΪ7NA��
�ʴ�Ϊ��sp3��sp2��N��O��C��H��7NA��
��3��MgO�����Ӷ���2����λ��ɣ�NaCl�����Ӷ���1����λ��ɣ����Ӱ뾶Cl��>O2����Mg2����Na�����ۻ�����ľ�����Զ���ڵͼ����ӻ�����ľ�������MgO��NaCl�����۵�MgO��NaCl���ʴ�Ϊ��MgO�����������Ӱ뾶С�����ӵ�����࣬�����ܴ�
��4��ԭ�ӷ�������Ϊ��0.5��0.2��0.5����Csԭ��λ�ھ������ڣ�ԭ�ӷ�������Ϊ��0��0.3��0.5������1.0��0.3��0.5����Csԭ�ӷֱ�λ�ھ���������桢�Ҳ����ϣ�ԭ�ӷ�������Ϊ��0.5��0.8��1.0������0.5��0.8��0����Csԭ�ӷֱ�λ�ھ������ϵ��桢�µ��棬ԭ�ӷ�������Ϊ��0��0.7��1.0������1.0��0.7��1.0����0��0.7��0������1.0��0.7��0����Csԭ��λ�ھ���ƽ����y������ϣ�����Csԭ����ĿΪ��1+4��![]() +4��
+4��![]() =4���ʴ�Ϊ4��
=4���ʴ�Ϊ4��

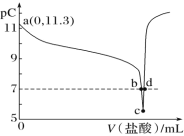
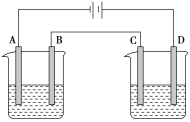
����Ŀ����ͼ���е��ʵ��(A��B��C��D��Ϊ���Ե缫)���ɹ�ѡ��ĵ������Һ�����±��С�Ҫ������������ǣ�
�ٹ���һ��ʱ��������Һ��pH������������Һ��pH�½���
��B��C�����Ϸŵ�����ӵ����ʵ�����ȡ�
�� ������������ ��
��� | �� | �� | �� | �� |
���ձ� | NaOH��Һ | NaCl��Һ | H2SO4��Һ | AgNO3��Һ |
���ձ� | CuSO4��Һ | AgNO3��Һ | AgNO3��Һ | CuCl2��Һ |
(1)Ӧѡ��ĵ������Һ��________�顣
(2)�缫��Ӧʽ��B��______________________��D��___________________________��
(3)��B��������3.55g������ʱ��C��������______�����������ƣ�������Ϊ______g��
����Ŀ��ʳ�ð״���Ч�ɷ�Ϊ���ᣨCH3COOH������ʹ������к͵ζ������вⶨ�����ñ�NaOH��Һ�ⶨ���ۡ��Ϻ��״ס�����������g/100mL��������дʵ�鱨���еĿհ״���
��ʵ��Ŀ�ģ��ⶨ���ۡ��Ϻ��״ס���������
��ʵ����Ʒ������ˮ���Ϻ��״ף�0.1000mol/LNaOH����Һ����̪��100mL����ƿ��___________�����������ζ��ܣ���ƿ���ձ�������̨��������
��ʵ�鲽�裩
��1�����Ʋ���ȡ����ʳ����Һ��
����___________�����������ƣ���ȡ10.00mLʳ�ð״ף�ע���ձ�����ˮϡ�ͺ�ת�Ƶ�100mL����ƿ�ж��ݣ�ҡ�ȼ��á�
��ȡ����״���Һ20.00 mL����ƿ�У����μ�2�η�̪��ָʾ����
��2��ʢװ��NaOH��Һ���ζ��ܾ���ϴ�Ӻ�װ��NaOH����Һ��ʹҺ��λ��________________________________����¼�ζ��ܵij���������Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ____________mL��
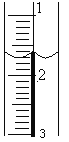
��3���ζ�����_____________________________________________ʱ��Ϊ�ζ��յ㡣�ζ������յ���¼NaOH��Һ���յ���������ظ��ζ�3�Ρ�
�����ݼ�¼�봦����
�ζ����� ʵ������ | 1 | 2 | 3 | 4 |
����ʳ�����/mL | 20.00 | 20.00 | 20.00 | 20.00 |
�ζ��ܳ�����/mL | 0.00 | 0.20 | 0.10 | 0.15 |
�ζ���ĩ����/mL | 15.95 | 16.20 | 15.15 | 16.20 |
��4����ͬѧ�ڴ�������ʱ����ã�����NaOH��Һ��ƽ�����(V)��![]() ��15.76 mL��ָ������IJ�����֮����____________________________________________��
��15.76 mL��ָ������IJ�����֮����____________________________________________��
��5������ȷ���ݴ������㣬���Ϻ��״ס�����������___________g/100mL��
��˼�������ۣ�
��6���鿴ƿʾ�������5g/100mL���Դ���ƣ�����˴β���������������Ϊ________��
��7�����в����п�����ɸò���������____________������ţ���
a. δ�ñ�NaOH��Һ��ϴ�ζ��� b. ��ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
c. �ζ�ǰδ���ߵζ��ܼ����е����� d. ��ƿ�м������״���Һ���ټ�����ˮ