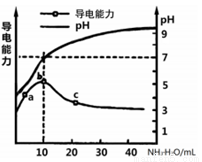
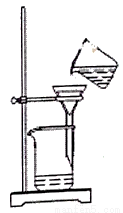
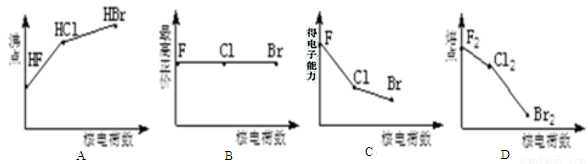
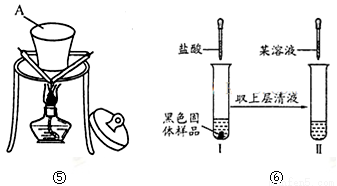
��Ŀ����
�����ʯ����Ȼ������ʯ������ͨ�����·�Ӧ����Ӧ��������֪���Ļ�ѧʽ������A~J����ѧ��ѧ�еij������ʣ�G��һ�ֺ��ɫ���壬D��һ��������ˮ�ĺ����ᣬJ��һ����Ҫ���������壬A����ɫ��Ӧ�ʻ�ɫ��E��B�ֱ����ữ����������Һ��Ӧʱ��ʵ��������ͬ�����а�ɫ������������ʵ���������ʯ��Ħ������Ϊ204g ��mol��1��������Ԫ�ص���������Ϊ54.9%�������еIJ�������������ȥ��
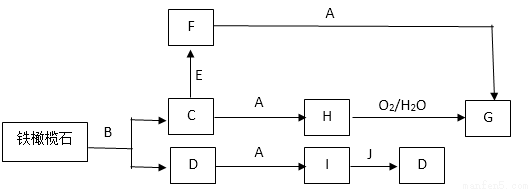
�ش��������⣺
��1��A�ĵ���ʽ_____________��
��2�������ʯ�Ļ�ѧʽ_____________��
��3��д��C��F�����ӷ���ʽ_____________��
��4��д��H��G�Ļ�ѧ����ʽ_____________��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ