��Ŀ����
��˹������Ϊ���������غ��,���ܻ�ѧ��Ӧ������һ����ɻ�ּ������,�������̵���ЧӦ����ͬ�ġ�
��֪:��H2O(g)=H2O(l) ��H1=-Q1 kJ/mol
��C2H5OH(g)=C2H5OH(l) ��H2=-Q2 kJ/mol
��C2H5OH(g)+3O2(g)=2CO2(g)+3H2O(g)��H3=-Q3 kJ/mol
��ʹ23 gҺ̬��ˮ�ƾ���ȫȼ��,���ָ�������,��ų�������Ϊ(��λ:kJ)�� ��
��֪:��H2O(g)=H2O(l) ��H1=-Q1 kJ/mol
��C2H5OH(g)=C2H5OH(l) ��H2=-Q2 kJ/mol
��C2H5OH(g)+3O2(g)=2CO2(g)+3H2O(g)��H3=-Q3 kJ/mol
��ʹ23 gҺ̬��ˮ�ƾ���ȫȼ��,���ָ�������,��ų�������Ϊ(��λ:kJ)�� ��
| A��Q1+Q2+Q3 | B��1.5Q1-0.5Q2+0.5Q3 |
| C��0.5Q1-1.5Q2+0.5Q3 | D��0.5(Q1+Q2+Q3) |
B
���ݸ�˹���ɢ١�3-��+�۵�,C2H5OH(l)+3O2(g)=3H2O(l)+2CO2(g) ��H=(-3Q1+Q2-Q3)kJ/mol=-(3Q1-Q2+Q3)kJ/mol,23 gҺ̬��ˮ�ƾ���0.5 mol��ȫȼ�շų�������Ϊ(3Q1-Q2+Q3)��0.5 kJ=(1.5Q1-0.5Q2+0.5Q3)kJ,��B��ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CH3OCH3��H2O
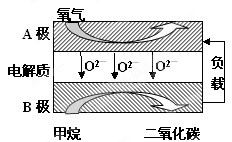
CH3OCH3��H2O CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol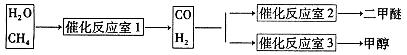

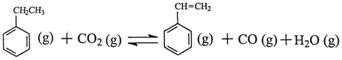 ��H
��H ��H1=��117.6kJ��mol��1
��H1=��117.6kJ��mol��1 CO (g)��H2O (g) ��H2=��41.2kJ��mol��1
CO (g)��H2O (g) ��H2=��41.2kJ��mol��1 ��
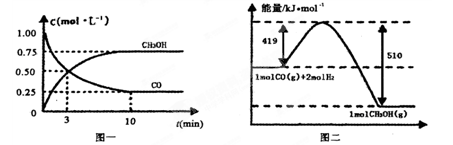
�� ����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú�
����Ӧ�ﵽƽ��ʱ����ϩ��Ũ��Ϊ �� �����ú� CO2(g) + 3H2(g) ����H>0
CO2(g) + 3H2(g) ����H>0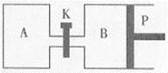

 2H2��+O2��
2H2��+O2�� 2H2��+O2��
2H2��+O2�� 2H2��+O2��
2H2��+O2�� CO+3H2
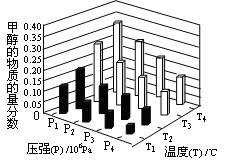
CO+3H2 CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
CH3OH��g��+H2O��g������H="-49.0" kJ/mol,���CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��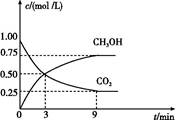
 CH3OH��g����ƽ�ⳣ��:
CH3OH��g����ƽ�ⳣ��:

 Fe2O3(s)+ CO(g)
Fe2O3(s)+ CO(g)
 Fe(s)+ CO2(g) ��H��-23.5 kJ��mol-1���÷�Ӧ��
Fe(s)+ CO2(g) ��H��-23.5 kJ��mol-1���÷�Ӧ��