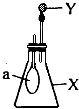��Ŀ����
����Ŀ������������NaOH��Һ���кͷ�Ӧû�����Ե�����ijѧϰ��ȤС���ͬѧΪ��֤��NaOH��Һ����������ᷢ���˷�Ӧ�����кͷ�Ӧ����ЧӦ��������������漸��ʵ�鷽������ش��й����⣺
��1������һ����ͼװ��ʵ��װ�ã�ͼ��С�Թ���ϸ�ߵ��ţ�ϸ�ߵ��϶�˩��ϸ��˿�ϡ���ʼʱʹ�Ҷ�U�ι����˺�īˮ��ƽ��ʵ�鿪ʼ�����²�ϸ��˿��ʹС�Թ���������ƿ��NaOH��Һ��ϣ���ʱ�۲쵽��������________________________________��ԭ����______________________________

��2������������С�������Ӧ��Һ�¶ȵı仯���жϷ�Ӧ�ķ��������NaOH��Һ��������ǰ�����¶ȵı仯����֤�������˻�ѧ��Ӧ����С��ͬѧ����ͬŨ�ȵ�NaOH��Һ�������10 mL��ϣ����¶ȼƲ�����Ӧǰ���¶ȵı仯����õIJ����������±���

��x��______
��3����HNO3(aq)��NaOH(aq)=NaNO3(aq)��H2O(l)����H����57.3 kJ��mol��1�����У���ϡH2SO4��Ba(OH)2(aq)����ŨH2SO4��Ba(OH)2(aq)����ϡHNO3��Ba(OH)2(aq)��Ӧ����1 mol H2O(l)�ķ�Ӧ�ȷֱ�Ϊ��H1����H2����H3������H1����H2����H3������С����Ĺ�ϵΪ______________________
���𰸡� U�ι���Һ������½����ұ����� �����NaOH�����кͷ�Ӧ�ų�������ʹƿ�������¶����ߣ�ѹǿ���� 7 ��H2����H1����H3
��������������Ҫ��������кͷ�Ӧ��̽��ʵ�顣
��1������һ��ʵ�鿪ʼ�����²�ϸ��˿��ʹС�Թ���������ƿ��NaOH��Һ��ϣ���ʱ�۲쵽��������U�ι���Һ������½����ұ�������ԭ���������NaOH�����кͷ�Ӧ�ų�������ʹƿ�������¶����ߣ�ѹǿ������
��2�����������������ķ�Ӧ������ʵ����뷴Ӧ�ȳ����ȣ������Ʋ�x��7��
��3��Ũ����ϡ�ͷų��϶��������������ᱵ�������ȣ�����H1����H2����H3������С����Ĺ�ϵΪ��H2����H1����H3��

 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�����Ŀ���±��ж���������ʵķ���ȫ����ȷ����
ѡ�� | ������ | ����� | ���������� | �ǵ���� |
A. | Һ�� | Ư�� | Al2O3 | CO |
B. | ������ˮ | ������������ | Na2O2 | �Ҵ� |
C. | ��ʯ�� | ���� | K2O | Cl2 |
D. | ���� | ˮ���� | CaO | SO2 |
A. A B. B C. C D. D