��Ŀ����
�����������ɵ����������ϳɰ����ķ��������1918��ŵ������ѧ�����ֽ������͵�������ij�ܱ������У���һ�������·�Ӧ���й�����Ϊ��
| ��Ŀ | H2 | N2 | NH3 |
| ��ʼʱ | 5 mol��L��1 | 3 mol��L��1 | 0 |
| 2 sĩ | 2 mol��L��1 | | |
(1)�����͵�����Ӧ���ɰ���(��2 s��)�ķ�Ӧ����v(H2)��__________������ʱ�Ѵ�ƽ�⣬������ƽ�ⳣ��Ϊ__________��
(2)��ͼ��ʾ�ϳ�NH3��Ӧ��ʱ��t0��t6�з�Ӧ�����뷴Ӧ��������ͼ���������дﵽ��ѧƽ���ʱ����У���ѧƽ�ⳣ������һ��ʱ����__________��
��t0��t1����t2��t3����t3��t4����t5��t6
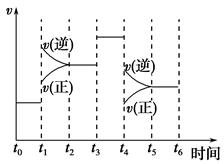
��t1ʱ�ı�������������¶ȣ���˵���ϳ�NH3��Ӧ���ʱ䦤H________0(����ڡ���С�ڡ�)��
(1)1.5 mol��L��1��s��1��0.25��(2)�١�С��
����

��ϰ��ϵ�д�
�����Ŀ
��һ��������ܱ�������,�������»�ѧ��Ӧ:CO2(g)+H2(g) CO(g)+H2O(g),�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ:
CO(g)+H2O(g),�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���ʾ:
| t/�� | 700 | 800 | 830 | 1 000 | 1 200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش���������:
(1)�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=��������
(2)�÷�ӦΪ��������Ӧ(����ȡ����ȡ�)��
(3)���жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������(����)
A.������ѹǿ����
B.���������c(CO)����
C.v��(H2)=v��(H2O)
D.c(CO2)=c(CO)
(4)ij�¶���,ƽ��Ũ�ȷ�����ʽ:c(CO2)��c(H2)=c(CO)��c(H2O),���жϴ�ʱ���¶�Ϊ������ �档
��һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У�������Ũ����ʱ��仯�Ĺ�ϵ��ͼ1��ʾ��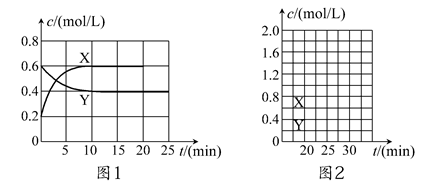
��ش�
(1)ͼl�У�����________(�X����Y��)��ʾNO2Ũ����ʱ��ı仯�����ǰ10 min��v(NO2)��________mol/(L��min)��
(2)����ѡ���в���˵���÷�Ӧ�Ѵﵽƽ��״̬����________(��ѡ����ĸ)��
| A�������ڻ�������ѹǿ����ʱ��仯���ı� |
| B�������ڻ��������ܶȲ���ʱ��仯���ı� |
| C�������ڻ���������ɫ����ʱ��仯���ı� |
| D�������ڻ�������ƽ����Է�����������ʱ��仯���仯 |
(4)��Ӧ���е�20 minʱ�����������ڳ���һ����NO2,10 min��ﵽ�µ�ƽ�⣬��ʱ���c(NO2)��0.9 mol/L��
�ٵ�һ��ƽ��ʱ���������NO2���������Ϊ��1���ﵽ��ƽ�����������NO2���������Ϊ��2�����1________��2(�>������������<��)��
������ͼ2�л���20 min������ʵ�Ũ����ʱ��仯������(�����ϱ�������X���͡�Y��)��
 [Cu(NH3)3]CH3COO��CO(����ӦΪ���ȷ�Ӧ)
[Cu(NH3)3]CH3COO��CO(����ӦΪ���ȷ�Ӧ)
 CH3OH(g)��H2O(g)�����ܱ������£�����ʾ��ͼ��˵����Ӧ���е�t1ʱ��ʱ�ﵽƽ��״̬���� (����ĸ���)
CH3OH(g)��H2O(g)�����ܱ������£�����ʾ��ͼ��˵����Ӧ���е�t1ʱ��ʱ�ﵽƽ��״̬���� (����ĸ���)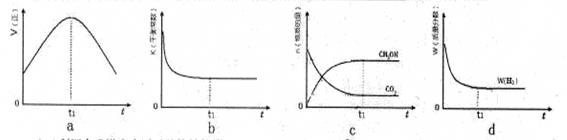
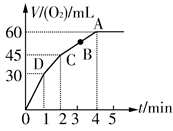
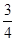 �����ʱ��ԼΪ__________________
�����ʱ��ԼΪ__________________ Si3N4(s)��12HCl(g)����H<0
Si3N4(s)��12HCl(g)����H<0 2HI(g)�Ѵ�ƽ��״̬��������������
2HI(g)�Ѵ�ƽ��״̬�������������� 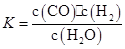 �����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬��������( )
�����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬��������( )