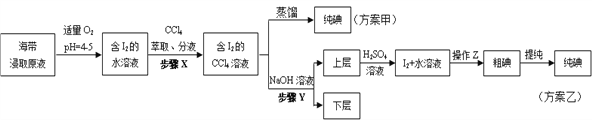
��Ŀ����
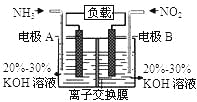
����Ŀ��ij100mL��Һ���ܺ���Na+��NH4+��Fe3+��CO32-��SO42-��Cl-�е������֣�ȡ����Һ��������ʵ�飬ʵ�������ͼ���������Լ�������������ȫ���ݳ�������˵������ȷ����

A. ԭ��Һһ������CO32����SO42����һ��������Fe3+
B. ԭ��Һһ������Cl�������ܴ���Na+
C. ԭ��Һ��c(CO32��) ��0.1mol��L-1
D. ��ԭ��Һ�в�����Na+����c(Cl��)��0.1mol��L-1
���𰸡�D
�������������Ȼ�����Һ�����ɳ�����һ������̼�����������е�����һ�֣���ó���ΪBaSO4��BaCO3�е�����һ�֣����������ܽ������ᣬ����һ����BaSO4��BaCO3�Ļ���һ������CO32����SO42�������ᱵ������2.33g�����ʵ�����2.33g/233g��mol��1=0.01mol��̼������ӵ����ʵ�����(4.3-2.33)/197mol=0.01mol��̼����������Ӳ����棬һ��������Fe3�������õ�����Һ�м����������ƣ��������壬Ϊ������һ������笠����ӣ�����Ԫ���غ㣬笠����ӵ����ʵ�����1.12L/22.4L��mol��1=0.05mol�����ݵ���غ㣬��������������ɵ����ʵ���֮�ͣ�0.05mol����������������ɵ����ʵ���֮��=0.01��2+0.01��2=0.04mol������һ�����������ӣ������Ӳ���ȷ����n��Cl������0.01mol������c��Cl������0.1mol��L-1��A��ԭ��Һһ������CO32����SO42����Cl����һ��������Fe3������A��ȷ��B��ԭ��Һһ������Cl�������ܴ���Na������B��ȷ��C��ԭ��Һ��c��Cl������0.1mol��L-1����C��ȷ��D����ԭ��Һ�в�����Na������c��Cl����=0.1mol��L-1����D����ѡD��

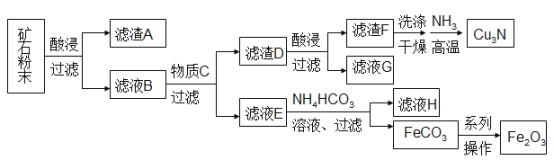
 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�