��Ŀ����
��ͼ��Ԫ�����ڱ���һ���֣�A��B��C��D��E��X�����ڱ�����Ԫ����ɵij������ʻ��
|
�� |
|
|
|
|
|
|
|
|
|||||||||
|
|
|
|
�� |
�� |
�� |
|
|
||||||||||
|
�� |
|
|
|
|
|
|
�� |
|
|||||||||
|
|
|
|
|
|
|
|
Fe |
|
|
|
|
|
|
As |
|
|
|
I��Ԫ�����ڱ��������о��������ʵ���Ҫ����
Y�ɢڢޢ�����Ԫ����ɣ�����ˮ��Һ�������г�������������As����Y��ˮ��Һ��Ӧ��������As����ۺ����ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ ��������1mol��ԭ��ʱ������ת���� ________mol��
����֪A��B��C��D��E��X������ͼ��ʾת����ϵ������������ͷ�Ӧ������ȥ��
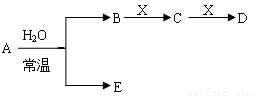
��1����EΪ�������A�Ļ�ѧʽΪ ��A��ˮ��Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ ��
�� ��X�Ǽ�������Һ��C��������22������ʱ����C�ĽṹʽΪ ����ʾX�ʼ��Ե����ӷ���ʽΪ ��
�� ��XΪ��������ʱ����X��B��ϡ��Һ��Ӧ����C�����ӷ�Ӧ����ʽΪ
��2����EΪ�������壬DΪ��ɫ������A�Ļ�ѧʽ������ ��B���еĻ�ѧ����Ϊ ��C��X��Ӧ�����ӷ���ʽΪ ��
��3����BΪ�������壬D����ˮ������һ�������·������淴Ӧ������C��һ�ֿ�ȼ�����嵥�ʣ���ÿ��淴Ӧ�Ļ�ѧ����ʽΪ ��t��ʱ�����ܱպ��ݵ�ij������Ͷ������ʵ�����D��ˮ������һ��ʱ����ƽ�⣬���¶��·�Ӧ��ƽ�ⳣ��K=1��D��ת����Ϊ ��
I 5NaClO + 2As +3H2O = 2H3AsO4 +5NaCl �� 5
II
��1��NO2��3NO2 +H2O = 2HNO3 + NO
��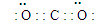 ��
��  ��H2O
��H2O 
 ��OH-
��OH-
�� Fe + 4H+ + =Fe3+ + NO��+2H2O
=Fe3+ + NO��+2H2O
��2��Na ��Na2O2 ��NaH�� ���Ӽ��ͼ��Թ��ۼ���
Al3+��3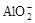 ��6H2O �� 4Al(OH)3��
��6H2O �� 4Al(OH)3��
��3��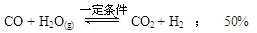 ��
��
��������








