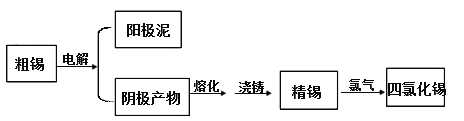
��Ŀ����
����Ŀ��һ����ȡ�����ƵĻ�����������ͼ��ʾ��
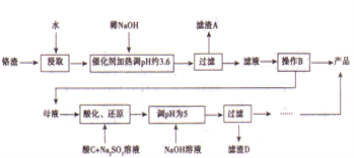
��֪��
�ٸ�������Na2S04������Cr2O72-��Fe3+����Fe3+��Cr3+ǡ����ȫ������c��1.0��10-5mol/L��ʱpH�ֱ�Ϊ3.6 ��5��
(1)����AΪ_______(�ѧʽ)��
(2)������ͼ�ܽ��(S)-�¶�(T)���ߣ�����B����ѷ���Ϊ_________(����ĸ���)��
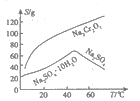
a������Ũ�������ȹ��� b������Ũ�������½ᾧ������
(3)�ữ��Cr2O72-�ɱ�SO32-��ԭ��Cr3+�����ӷ���ʽΪ____________����CΪ______________��Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]=______________��
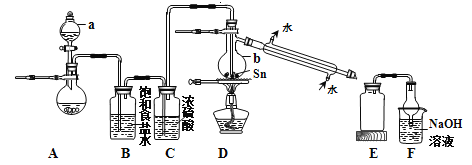
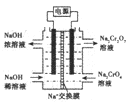
(4)���ݷ�Ӧ2CrO42-+2H+![]() Cr2O72-+H2O �����ͼ��ʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��____________������缫��ӦʽΪ____________��ͨ��2mol���ӣ�����Cr2O72-�����ʵ�����__________________��
Cr2O72-+H2O �����ͼ��ʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��____________������缫��ӦʽΪ____________��ͨ��2mol���ӣ�����Cr2O72-�����ʵ�����__________________��
���𰸡���Fe(OH)3
��a
��3SO32-��Cr2O72-��8H+=2Cr3+��3SO42-��4H2O H2SO4 1.0��10-32
���� 4OH��-4e��=O2����2H2O 1mol
�����������⿼�黯ѧ�������̣���1����Ϊ����pH��3.6����ʱFe3��������������ʽ��ȫ�������������AΪFe(OH)3����2�����̵�Ŀ������ȡNa2SO4�������ܽ�ȣ��¶����ߣ�Na2SO4���ܽ�������¶����߶����ͣ���˲�������Ũ�������ȹ��ˣ��õ�Na2SO4������ѷ�����ѡ��a����3��Cr2O72����Cr�Ļ��ϼ��ɣ�6�ۡ���3�ۣ����ϼ۹�����3��2=6�ۣ�SO32����S�ļ�̬�ɣ�4�ۡ���6�ۣ����ϼ�����2�ۣ���С������Ϊ6��������ӷ�Ӧ����ʽΪ3SO32����Cr2O72����8H��=2Cr3����3SO42����4H2O ����ΪCr2O72������ǿ�����ԣ��������������������Լ�������лӷ��ԣ�����ữCr2O72�����õ�����H2SO4��Cr3����ȫת���ɳ�����pH=5������Һ��c(OH��)=10��9mol��L��1��Ksp[Cr(OH)3]=c(Cr3��)��c3(OH��)=10��5��(10��9)3=10��32����4��Cr�Ļ��ϼ�û�з����仯��������ʾ�ĵ��װ��ͼ��������ϡ����������Һ����������Ũ����������Һ���˵缫�ϲ���OH�����õ�H2�����˼�Ϊ�������Ҳ�Ϊ�������ӵ�Դ����������缫��ӦʽΪ4OH����4e��=O2����2H2O��2H2O��4e��=O2����4H����ͨ��2mol���ӣ�����2molH����������Cr2O72�������ʵ���Ϊ1mol��

 ѧ���쳵��������������������ϵ�д�
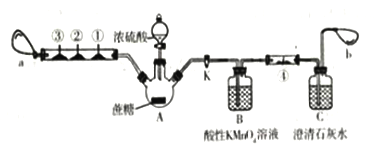
ѧ���쳵��������������������ϵ�д�����Ŀ��ʵ���У���ѡȡ�ķ���װ�����Ӧԭ������ȷ���� �� ��
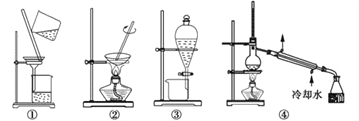
ѡ�� | Ŀ �� | װ�� | ԭ �� |
A | ������������Ĵ��� | �� | �������Ӳ���ͨ����ֽ�����Ӽ�С���ӿ���ͨ����ֽ |
B | ���뱽�е��屽 | �� | ��(0.88 g/mL)���屽(1.5 g/mL)���ܶȲ�ͬ |
C | �����ᴿ | �٢� | NaCl��ˮ�е��ܽ�Ⱥܴ� |
D | ��ȥ�������еĻ�ϩ | �� | �������ķе�(161 ��)�뻷��ϩ�ķе�(83 ��)���ϴ� |
A. A B. B C. C D. D