��Ŀ����
��10�֣�ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ������¼���£�
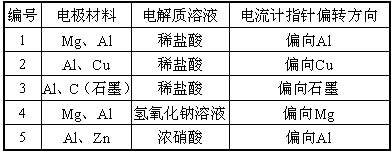
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��C(ʯī) | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | ����������Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
(1)ʵ��1��2��Al�����ĵ缫(������)�Ƿ���ͬ(���ͬ������ͬ��)________��
(2)��ʵ��3���������գ�
����Ϊ________�����缫��Ӧʽ��______________________________��
��ʯīΪ________�����缫��Ӧʽ��___________________________��
�۵���ܷ�Ӧʽ��______________________________________��
(3)ʵ��4������������������________��������__________________________
д�����缫�ĵ缫��Ӧʽ_________________________________��
(4)����ʵ��5�е�����ָ��ƫ������ԭ��_____________________________��
(1)����ͬ
(2)�ٸ���2Al��6e��===2Al3�� ������6H����6e��===3H2�� ��2Al��6HCl===2AlCl3��3H2��
(3)����������������������Һ����������ԭ��Ӧ����þ��������������Һ������Ӧ
Al��3e����4OH��===AlO2-��2H2O
(4)����Ũ�����б��ۻ���п��Ũ�����б�����������Ũ������Zn��ԭ��صĸ�����Al��ԭ��ص����������Ե�����ָ��ƫ����
���������������1��ʵ��1��Mg��Al���ã�Al��������ʵ��2��Al��Cu���ã�Al�����������Բ���ͬ��
��2�����ݵ缫�����ж�Al�Ǹ������缫��ӦΪ2Al��6e-=2Al3����ʯī���������缫��ӦΪ6H����6e-=3H2��������ܷ�ӦΪ2Al��6HCl===2AlCl3��3H2��
��3��Al����NaOH��Һ��Ӧ��Mg���ܣ�������NaOH��Һ��Al��Mg���ã�����Al���������缫��ӦΪAl��3e����4OH��===AlO2-��2H2O��
��4������Ũ�����б��ۻ���п��Ũ�����б�����������Ũ������Zn��ԭ��صĸ�����Al��ԭ��ص����������Ե�����ָ��ƫ������
���㣺ԭ���
��������ԭ����нϻ��õĽ�����������ʧȥ���ӣ�����������Ӧ�����Ӿ����ߴ��ݵ������ϣ�������Һ�е��������������ƶ������������ƶ��������õ����ӣ�������ԭ��Ӧ���ݴ˿��Խ����йص��жϡ�

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д���10�֣�ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ������¼���£�
|
��� |
�缫���� |
�������Һ |
������ָ��ƫת���� |
|
1 |
Mg��Al |
ϡ���� |
ƫ��Al |
|
2 |
Al��Cu |
ϡ���� |
ƫ��Cu |
|
3 |
Al��C(ʯī) |
ϡ���� |
ƫ��ʯī |
|
4 |
Mg��Al |
����������Һ |
ƫ��Mg |
|
5 |
Al��Zn |
Ũ���� |
ƫ��Al |
�Ը��ݱ��е�ʵ������ش��������⣺
(1)ʵ��1��2��Al�����ĵ缫(������)�Ƿ���ͬ(���ͬ������ͬ��)________��
(2)��ʵ��3���������գ�
����Ϊ________�����缫��Ӧʽ��______________________________��
��ʯīΪ________�����缫��Ӧʽ��___________________________��
�۵���ܷ�Ӧʽ��______________________________________��
(3)ʵ��4������������������________��������__________________________
д�����缫�ĵ缫��Ӧʽ_________________________________��
(4)����ʵ��5�е�����ָ��ƫ������ԭ��_____________________________��