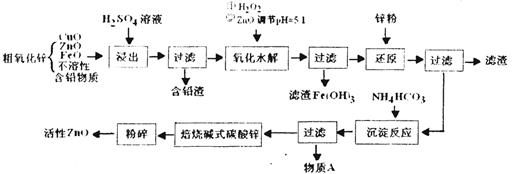
��Ŀ����
I��Ŀǰ���ҹ����á��Ӵ����������ᣬ�豸��ͼ��ʾ��
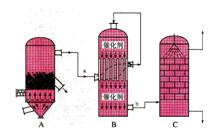
��1��ͼ���豸A��������_____________ ���豸����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��2���йؽӴ��������������˵���У�����ȷ����______________��
E�����Ṥҵ���ڽӴ��Ұ�װ�Ƚ�������Ϊ������SO3ת��ΪH2SO4ʱ�ų�������
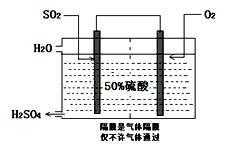
��3�����й����߿������Ʊ�SO2�����õ绯ѧԭ����������ķ�����װ����ͼ��Ϊ���ȶ����������������Ũ��Ӧά�ֲ��䣬��ͨ���SO2��ˮ��������Ϊ ��
II. ������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�� ��������͡������Ƽ�����ֹ��ա��밴Ҫ��ش����⣺
��1�����������������CaCl2�������д���ù����в���CaCl2�Ļ�ѧ����ʽ�� ��
��2��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��
��3��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ����CO2����Դ�� ��
�� �������CO2��Դ�� ��
�������CO2��Դ�� ��

��1��ͼ���豸A��������_____________ ���豸����Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��
��2���йؽӴ��������������˵���У�����ȷ����______________��
A����������ĽӴ������ڽӴ����з��� | B����������Ũ��Ϊ98.3%Ũ���������������� |
| C�����պ���48%�Ļ�����ʱ����FeS2��ʧ��2%����S��ʧ2% | |
| D��Bװ���з�Ӧ������֮һΪ�ϸ��¶���Ϊ�����SO2��ת���� |
��3�����й����߿������Ʊ�SO2�����õ绯ѧԭ����������ķ�����װ����ͼ��Ϊ���ȶ����������������Ũ��Ӧά�ֲ��䣬��ͨ���SO2��ˮ��������Ϊ ��

II. ������һ����Ҫ�Ļ���ԭ�ϡ�Ŀǰ�Ƽҵ��Ҫ�� ��������͡������Ƽ�����ֹ��ա��밴Ҫ��ش����⣺
��1�����������������CaCl2�������д���ù����в���CaCl2�Ļ�ѧ����ʽ�� ��
��2��д���������Ƽ���йط�Ӧ�Ļ�ѧ����ʽ�� ��
��
��3��CO2���Ƽҵ����Ҫԭ�ϣ��������Ƽ����CO2����Դ�� ��
��
 �������CO2��Դ�� ��
�������CO2��Դ�� ����1������¯ ��1�֣���4FeS2 +11O2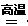 2Fe2O3 + 8SO2��2�֣�
2Fe2O3 + 8SO2��2�֣�
��2��D E��2�֡����ֱ�ͬǰ�� ��3��16�s29 ��2�֣�
��1��2NH4Cl + Ca(OH)2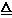 CaCl2 + 2NH3��+ 2H2O ��2�֣�
CaCl2 + 2NH3��+ 2H2O ��2�֣�
��2��NH3 + H2O + CO2 + NaCl�����ͣ� �� NaHCO3��+ NH4Cl��2�֣�
2NaHCO3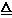 Na2CO3 + CO2��+ H2O ��2��)
Na2CO3 + CO2��+ H2O ��2��)
��3���ϳɰ�����1�֣� ������ʯ��ʯ��1�֣�
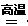 2Fe2O3 + 8SO2��2�֣�
2Fe2O3 + 8SO2��2�֣���2��D E��2�֡����ֱ�ͬǰ�� ��3��16�s29 ��2�֣�
��1��2NH4Cl + Ca(OH)2
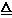 CaCl2 + 2NH3��+ 2H2O ��2�֣�
CaCl2 + 2NH3��+ 2H2O ��2�֣���2��NH3 + H2O + CO2 + NaCl�����ͣ� �� NaHCO3��+ NH4Cl��2�֣�
2NaHCO3
 Na2CO3 + CO2��+ H2O ��2��)
Na2CO3 + CO2��+ H2O ��2��)��3���ϳɰ�����1�֣� ������ʯ��ʯ��1�֣�
��

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ

 ѹǿ
ѹǿ �Ŀ�����Ϊ��________��
�Ŀ�����Ϊ��________��

 4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ���
4Fe(OH)3+8OH��+3O2�����ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ��_______��Һ������ţ��� ���̿��Ʊ�������ص�һ�ֹ������̡�
���̿��Ʊ�������ص�һ�ֹ������̡�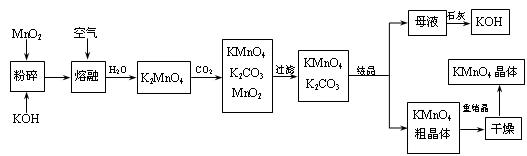
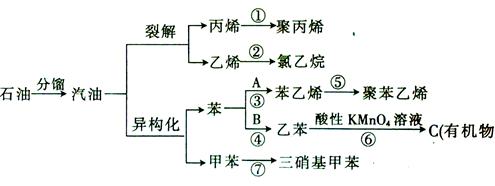
 ____________________________________________��
____________________________________________��