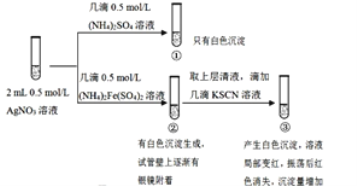
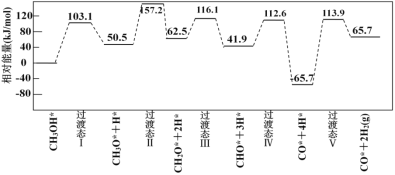
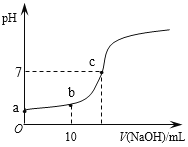
��Ŀ����
����Ŀ���ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�飬������ԭ�ζ�ʵ��������к͵ζ����ơ���Ѫ�Ƶĺ���ʱ����������ʵ�飺
�ٿɽ�4mLѪҺ������ˮϡ�ͺ������м������������(NH4)2C2O4���壬��Ӧ����CaC2O4��������������ϡ���ᴦ����H2C2O4��Һ��
�ڽ��ٵõ���H2C2O4��Һ����������KMnO4��Һ�ζ�����������ΪCO2����ԭ����ΪMn2+��
���յ�ʱ��ȥ20mL l.0��l0-4mol/L��KMnO4��Һ��
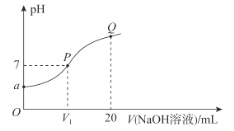
(1)д��KMnO4��Һ�ζ�H2C2O4��Һ�����ӷ���ʽ______________________________________���ζ�ʱ��KMnO4��ҺӦװ��______������������������ʽ�ζ����У��ζ��յ�ʱ�ζ�������_________________________��
(2)���в����ᵼ�²ⶨ���ƫ�͵���_________��
A���ζ�����װҺǰδ�ñ�KMnO4��Һ��ϴ
B���ζ������У���ƿҡ����̫���ң���ƿ����Һ�ν���
C���ζ��ܼ��첿���ڵζ�ǰû�����ݣ��ζ��յ�ʱ��������
D���ﵽ�ζ��յ�ʱ�����Ӷ���
(3)���㣺ѪҺ�к������ӵ�Ũ��Ϊ_________mol/L��
���𰸡�2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O �� �������һ��KMnO4��Һʱ����Һ����ɫ��Ϊdz��ɫ������Ӳ��ָ�ԭɫ BC 1.25��10-3
��������
(1)���������Һ����ǿ�����ԣ��ܽ�����������CO2����������ԭ��Mn2�������û��ϼ�������������ƽ��KMnO4����ǿ�����ԣ����ݵζ��ܵĹ��죬���Ը��������Һʢ�ŵ���ʽ�ζ��ܣ�������ɫ�������������(��)ɫ���ø��������Һ�ζ����ᣬ�Ӷ��ó��ζ��յ�����
(2)�����ζ������е���ע�����V��ı仯��
(3)���ݷ�Ӧ���̣�������ϵʽΪ5Ca2����5CaC2O4��5H2C2O4��2MnO4�������м��㣻
(1)KMnO4��H2C2O4������CO2����������ԭ��Mn2�������ݻ��ϼ�������������ƽ�������ӷ���ʽΪ2MnO4����5H2C2O4��6H��=2Mn2����10CO2����8H2O����ʽ�ζ��ܵ��¶��ж������������Һ����ǿ�����ԣ�����������˸��������ҺӦʢ������ʽ�ζ����У�������Һ��ɫ�����������Һ����(��)ɫ���ø��������Һ�ζ����ᣬ�յ�ʱ�ζ��������Ǽ������һ��KMnO4��Һʱ����Һ����ɫ��Ϊdz��ɫ�������(��30s)���ָ�ԭɫ��
(2)����2V��c(H2C2O4)=5V��c(MnO4��)�������
A. �ζ�����װҺǰδ�ñ�KMnO4��ϴ����ɱ�ҺŨ�Ƚ��ͣ����ı�Һ����������ⶨ���ƫ�ߣ���A���������⣻
B. ҡ����̫���ң���ƿ����Һ�ν�������������ʵ�����С�����ı�Һ�����С���ⶨ���ƫ�ͣ���B�������⣻
C. �ζ�ǰû�����ݣ��ζ��յ㷢�����ݣ����ı�Һ���ƫС���ⶨ���ƫ�ͣ���C�������⣻
D. �ζ��յ�ʱ�����Ӷ�����������Һ�����ƫ�ⶨ���ƫ�ߣ���D���������⣻
(3)���շ�Ӧ���̣�������ϵʽΪ5Ca2����5CaC2O4��5H2C2O4��2MnO4�����ó�c(Ca2��)=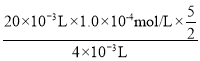 =1.25��10��3mol��L��1��
=1.25��10��3mol��L��1��

 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�