��Ŀ����
����Ŀ��ʳ������A��������֧����B����������B��ʹBr2��CCl4��Һ��ɫ��1mol B������Na��Ӧ����0.5mol H2�������ʼ�����ͼ1ת����
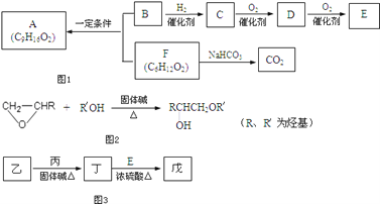
�ش��������⣺
��1����һ������������C������Ӧ����____��
a.Na b.Na2CO3 c.Br2/CCl4 d.CH3COOH
��2��D�����еĹ�����������____��E�Ľṹ��ʽ��____��
��3��B��F��Ӧ����A�Ļ�ѧ����ʽ��____��
��4��F��ij��ͬ���칹����ܷ���ˮ�ⷴӦ�����е�һ�ֲ����ܷ���������Ӧ����һ�����ڹ��������µ�һ��ȡ����ֻ�����֣��Ҵ�������IJ���Ҳ�ܷ���������Ӧ����Ľṹ��ʽ��____��
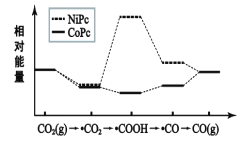
��5����֪����ij�����֮���ں����ȵĻ����»�����ͼ2��Ӧ���ӷ�Ӧ�����������÷�Ӧ���Ϳɳ�Ϊ____��
��6����ҵ�����ҳ������Ժϳ��л��ܼ��죨C7H14O3��������ͼʾ��ͼ3��
��֪��������C��ͬϵ�������������ڳ�����Ϊ��̬���ڶ���������2�������Ʋ���Ľṹ��ʽ��____��
���𰸡�ad ȩ�� CH3CH2COOH CH3(CH2)4COOH+CH2=CHCH2OH![]() CH3(CH2)4COOCH2CH=CH2+H2O HCOOCH2C(CH3)3 �ӳɷ�Ӧ CH3CH2COOCH(CH3)CH2OCH3
CH3(CH2)4COOCH2CH=CH2+H2O HCOOCH2C(CH3)3 �ӳɷ�Ӧ CH3CH2COOCH(CH3)CH2OCH3
��������
���л����ת����ϵ��֪��F����NaHCO3��Ӧ����CO2��Ӧ����-COOH����F����ʽ��֪���ڱ���һԪ���ᣬ�Ҳ�����֧����ӦΪCH3(CH2)4COOH��B��F��Ӧ����A���ɷ���ʽ��ֻB��Ӧ����3��Cԭ�ӣ�B����������B��ʹBr2��CCl4��Һ��ɫ��˵������̼̼˫����1mol B������Na��Ӧ����0.5mol H2��˵�������к���1��-OH����BӦΪCH2=CHCH2OH�� AΪCH3(CH2)4COOCH2CH=CH2��B�����������ӳɷ�Ӧ����C����CΪCH3CH2CH2OH��C��������D����DΪCH3CH2CHO��D��һ����������E����EΪCH3CH2COOH��
(1)CΪCH3CH2CH2OH�������ǻ��������ơ����ᷴӦ��������̼���ơ�������Ȼ�̼��Һ��Ӧ���ʴ�Ϊ��ad��
(2)DΪCH3CH2CHO�����й�����Ϊ��ȩ����E�Ľṹ��ʽ��CH3CH2COOH���ʴ�Ϊ��ȩ����CH3CH2COOH��
(3)B��F�ķ�ӦΪ��Ũ���������£�CH2=CHCH2OH��CH3(CH2)4COOH���ȷ���������Ӧ����CH3(CH2)4COO CH2CH=CH2����Ӧ�Ļ�ѧ����ʽ�ǣ�CH3(CH2)4COOH+CH2=CHCH2OH![]() CH3(CH2)4COO CH2CH=CH2+H2O���ʴ�Ϊ��CH3(CH2)4COOH+CH2=CHCH2OH
CH3(CH2)4COO CH2CH=CH2+H2O���ʴ�Ϊ��CH3(CH2)4COOH+CH2=CHCH2OH![]() CH3(CH2)4COOCH2CH=CH2+H2O��
CH3(CH2)4COOCH2CH=CH2+H2O��
(4)F�Ľṹ��ʽΪCH3(CH2)4COOH��F��ͬ���칹����ܷ���ˮ�ⷴӦ�������������ʣ����е�һ�ֲ����ܷ���������Ӧ��ӦΪ����������һ������ŨH2SO4�������������Ȳ��ܲ���ʹBr2��CCl4��Һ��ɫ���л��˵�����ܷ�����ȥ��Ӧ����-OH��λCԭ���ϲ���Hԭ�ӣ������������IJ���Ҳ�ܷ���������Ӧ��˵������-CH2OH������л���Ľṹ��ʽӦΪ��HCOOCH2C(CH3)3���ʴ�Ϊ��HCOOCH2C(CH3)3��
(5)�ӷ�Ӧ�����������÷�ӦΪ![]() ��
��![]() �����ӳɷ�Ӧ����
�����ӳɷ�Ӧ����![]() ���ʴ�Ϊ���ӳɷ�Ӧ��
���ʴ�Ϊ���ӳɷ�Ӧ��
(6)������C��ͬϵ����ڴ������ʣ�����������Ϊȩ�࣬�ڳ�����Ϊ��̬����Ϊ��ȩ���������Ǽ״���EΪCH3CH2COOH���붡��Ӧ�õ��죬������ķ���ʽ�����Ƶñ�Ϊ![]() ����ΪCH3OCH2/span>CH2OH����ΪCH3CH2COOCH(CH3)CH2OCH3���ʴ�Ϊ��CH3CH2COOCH(CH3)CH2OCH3��
����ΪCH3OCH2/span>CH2OH����ΪCH3CH2COOCH(CH3)CH2OCH3���ʴ�Ϊ��CH3CH2COOCH(CH3)CH2OCH3��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ�����Ԫ�������ɣ����������ʵ�Ʋⲻ�������ǣ���
��ʵ | �Ʋ� | |
A | IVA�� | ͬ�� |
B | HI��230��ʱ�ֽ⣬HCl��1500��ʱ�ֽ� | HF�ֽ��¶ȴ���1500�� |
C | H3PO4����ǿ�ᣬH2SO4��ǿ�� | HClO4��ǿ�� |
D | Na��Alͨ���õ�ⷨұ�� | Mg���õ�ⷨұ�� |
A.AB.BC.CD.D