��Ŀ����
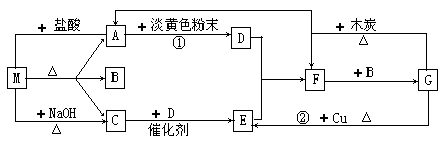
��֪A��B��C��D����ѧ��ѧ�ij������ʣ���A��B��C������ͬһ��Ԫ�ء���һ������������֮����ת����ϵ��ͼ��ʾ(���ַ�Ӧ�е�H2O����ȥ)��

��ش��������⣺
(1)��A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������____________________����ҵ����ȡA�����ӷ���ʽΪ______________________��
(2)��A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ________________________��
(3)��D���ȼҵ����Ҫ��Ʒ֮һ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ��____________________��
(4)��A��C��D���dz������壬C�ǵ����������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽΪ____________________��
ijͬѧ���Ѽ�����һ���������걣�����ܱ������У�ÿ��һ��ʱ��������pH����������ʼһ��ʱ���ڣ������pH�ʼ�С���ƣ������ӷ���ʽ����ԭ��_______________

��ش��������⣺
(1)��A����������ˮ������D�����������������������;���Ľ������ʣ���������B����Һ���ܵõ�B����B�Ļ�ѧʽ������____________________����ҵ����ȡA�����ӷ���ʽΪ______________________��
(2)��A��һ�ּ������壬�������������B������β��֮һ�����������ɫ����Ӧ�ٵĻ�ѧ����ʽΪ________________________��
(3)��D���ȼҵ����Ҫ��Ʒ֮һ��B�����ԣ���Ӧ�ڵ����ӷ���ʽ��____________________��
(4)��A��C��D���dz������壬C�ǵ����������Ҫ���壬��Ӧ�۵Ļ�ѧ����ʽΪ____________________��
ijͬѧ���Ѽ�����һ���������걣�����ܱ������У�ÿ��һ��ʱ��������pH����������ʼһ��ʱ���ڣ������pH�ʼ�С���ƣ������ӷ���ʽ����ԭ��_______________
(1)FeCl3��2Cl����2H2O 2OH����Cl2����H2��
2OH����Cl2����H2��
(2)4NH3��5O2 4NO��6H2O
4NO��6H2O
(3)Al(OH)3��OH��=AlO2����2H2O
(4)2H2S��SO2=3S����2H2O��2H2SO3��O2=4H����2SO42��
 2OH����Cl2����H2��
2OH����Cl2����H2��(2)4NH3��5O2
 4NO��6H2O
4NO��6H2O(3)Al(OH)3��OH��=AlO2����2H2O
(4)2H2S��SO2=3S����2H2O��2H2SO3��O2=4H����2SO42��
(1)��������������ˮ��������AΪCl2������ʹ����㷺�Ľ������ʣ�D��������BΪFeCl3��C��FeCl2��
(2)��������ΪNH3������β������������ɫ��������NO��
(3)����������������Al2O3��Al(OH)3��D��C�ֱ�ΪNaOH��NaAlO2����BΪAl(OH)3��
(4)�����������Ҫ������SO2��A��SO2��������Ԫ�أ��Ҷ��߷�Ӧ�������ʣ���AΪH2S���������Ҫ�ɷ���H2SO3���ڷ��ù����б�����ΪH2SO4��������ǿ��
(2)��������ΪNH3������β������������ɫ��������NO��
(3)����������������Al2O3��Al(OH)3��D��C�ֱ�ΪNaOH��NaAlO2����BΪAl(OH)3��
(4)�����������Ҫ������SO2��A��SO2��������Ԫ�أ��Ҷ��߷�Ӧ�������ʣ���AΪH2S���������Ҫ�ɷ���H2SO3���ڷ��ù����б�����ΪH2SO4��������ǿ��

��ϰ��ϵ�д�
�����Ŀ
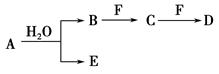
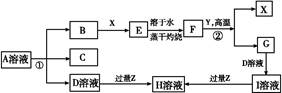
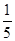 ����B��D�ֱ�����ˮ��������Һ��ǡ��������ϣ��ɵ�һ�ֲ�������Ԫ�ص�����Һ����д��A��H2O��Ӧ�Ļ�ѧ����ʽ��_____________________________________��
����B��D�ֱ�����ˮ��������Һ��ǡ��������ϣ��ɵ�һ�ֲ�������Ԫ�ص�����Һ����д��A��H2O��Ӧ�Ļ�ѧ����ʽ��_____________________________________��