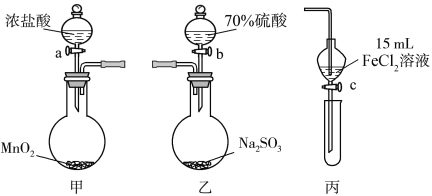
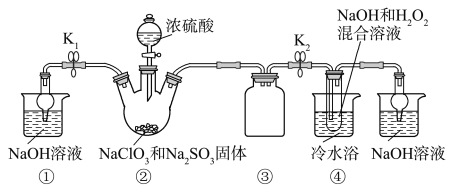
��Ŀ����
����Ŀ��1.52 gþͭ�Ͻ���ȫ�ܽ���50 mL�ܶ�Ϊ1.40 g��mL��1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������ 1 120 mL(��״��)����Ӧ�����Һ�м���1.0 mol��L��1NaOH��Һ������������ȫ������ʱ���õ�2.54 g������������������⣺
��1���úϽ���þ��ͭ�����ʵ���֮���� ��
��2��NO2��N2O4�Ļ�������У�NO2����������� ��
��3���õ�2.54 g����ʱ������NaOH��Һ������� mL��
���𰸡�(1)1��2 ��2��80% ��3��640 mL
��������
�����������1����Mg��Cu�����ᷴӦ�Ĺ����У���������+2�۵Ľ���������ʧȥ���ӵ����ʵ��������ᷴӦ�õ����ӵ����ʵ�����ȡ�������ΪMg2+��Cu2+������Ӧ�����Һ�м���������NaOH��Һʱ��������Ӧ�γ�Mg(OH)2��Cu(OH)2���������ӵ���������OH-��������m(OH-)=2.54g-1.52g=1.02g��n(OH-)=1.02g��17g/mol=0.06mol����n(Mg)+n(Cu)=0.03mol������Mg�����ʵ�����x��Cu�����ʵ�����y����x+y=0.03mol��24x+64y=1.52g�����x=0.01mol��y=0.02mol�����ԸúϽ���ͭ��þ�����ʵ���֮����0.02mol��0.01mol=2:1��
��2��������������ԭ��Ӧ�н���ʧȥ���ӵ����ʵ����������ᷢ����ԭ��Ӧ�õ����ӵ����ʵ��������ڷ�Ӧ�����ϵ����������ӵ����ʵ�����n(e-)=n(OH-)=0.06mol��NO2��N2O4�Ļ������1120 mL�����ʵ�����n=1.12L/22.4L/mol=0.05mol������NO2��N2O4�����ʵ����ֱ���a��b������ݵ����غ�ɵ�a+2b=0.06mol������Ԫ���غ�ɵ�a+b=0.05mol�����a=0.04mol��b=0.01mol������![]() ��֪������ͬ��������£����������ȵ�����������ʵ����ıȣ�����NO2��N2O4�Ļ�������У�NO2�����������(0.04mol��0.05mol)��100%= 80%��
��֪������ͬ��������£����������ȵ�����������ʵ����ıȣ�����NO2��N2O4�Ļ�������У�NO2�����������(0.04mol��0.05mol)��100%= 80%��
��3��50mL �ܶ�Ϊ1.40 g/mL����������Ϊ63%��Ũ�����к�����������ʵ����ǣ�n(HNO3)=cV=14mol/L��0.05L=0.7mol����ǡ���γɳ���ʱ����ҺΪNaNO3��Һ������Ԫ���غ㣬�ɵ�n(NaOH)=n(HNO3)(��)=0.7mol-0.04mol-0.01mol��2=0.64mol���ʵ��õ�2.54 g����ʱ������NaOH��Һ�������V=n/c=0.64mol��1.0 mol/L=0.64L��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�