��Ŀ����
3����֪A��B��C��D��E��F��G���Ƕ����ڵ�Ԫ�أ����ǵ�ԭ���������ε�����Aԭ�ӵĵ��Ӳ��������ĺ������������ͬ����Bԭ�ӵ������������Ǵ�����2����C���⻯���ʹʪ��ĺ�ɫʯ����ֽ������B��D�����γ�������̬�����Eԭ�Ӻ������������B��2����1��D��Fͬ���壬G��ԭ�Ӱ뾶�DZ�����Ԫ������С�ģ�����1��AԪ��ΪHCԪ��ΪNEԪ��ΪNa
��2��������Ԫ���γɵ�10���ӷ�����H2O��CH4��NH3��
��3����B��D��������Ϊ3��8��ɵĻ�������E��ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��4��G������E������������ˮ���ﷴӦ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O
��5��B��C��D�γɵ���̬�⻯���У����ȶ�����H2O
��6����A��C��G�γɵ����ӻ�����Ļ�ѧʽ��NH4Cl��
���� Aԭ�ӵĵ��Ӳ��������ĺ������������ֻͬ��H���⣩������������AΪ�⣻Bԭ�ӵ������������Ǵ�����2��������Ϊ���ǵ�ԭ���������ε���������Ҫ��С��ԭ���������ǣ�����Bԭ��ΪC��̼������������Ϊ4�������Ϊ2����������������BΪ̼��C���⻯���ʹʪ��ĺ�ɫʯ����ֽ��������������������ǰ���������CΪ����B��D�����γ�������̬���������ΪBΪ̼������DΪ����Eԭ�Ӻ������������B��2����1������EΪNa��D��Fͬ���壬����Ϊ���Ƕ����ڵ�Ԫ�أ�����F����G��ԭ�Ӱ뾶�DZ�����Ԫ������С�ģ�����Ϊ���ǵ�ԭ���������ε���������Gֻ���ǵ������ڣ�����G���ȣ�����������AΪH��BΪC��CΪN��DΪO��EΪNa��FΪS��GΪCl���ݴ˴��⣮
��� �⣺Aԭ�ӵĵ��Ӳ��������ĺ������������ֻͬ��H���⣩������������AΪ�⣻Bԭ�ӵ������������Ǵ�����2��������Ϊ���ǵ�ԭ���������ε���������Ҫ��С��ԭ���������ǣ�����Bԭ��ΪC��̼������������Ϊ4�������Ϊ2����������������BΪ̼��C���⻯���ʹʪ��ĺ�ɫʯ����ֽ��������������������ǰ���������CΪ����B��D�����γ�������̬���������ΪBΪ̼������DΪ����Eԭ�Ӻ������������B��2����1������EΪNa��D��Fͬ���壬����Ϊ���Ƕ����ڵ�Ԫ�أ�����F����G��ԭ�Ӱ뾶�DZ�����Ԫ������С�ģ�����Ϊ���ǵ�ԭ���������ε���������Gֻ���ǵ������ڣ�����G���ȣ�����������AΪH��BΪC��CΪN��DΪO��EΪNa��FΪS��GΪCl��
��1����������ķ�����֪��AΪH��CΪN��EΪNa��
�ʴ�Ϊ��H��N��Na��
��2��AΪH��BΪC��CΪN��DΪO��EΪNa��FΪS��GΪCl��������Ԫ���γɵ�10���ӷ�����H2O��CH4��NH3��
�ʴ�Ϊ��H2O��CH4��NH3��
��3��C��O�γ�������Ϊ3��8�Ļ������У�Cԭ������Oԭ����֮��Ϊ=1��2��������ʽΪCO2����Mg�ܷ�����Ӧ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
�ʴ�Ϊ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
��4��EΪNa��GΪCl��G������E������������ˮ���ﷴӦ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
��5��Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ����ǽ�����C��N��O������B��C��D�γɵ���̬�⻯���У����ȶ�����H2O��
�ʴ�Ϊ��H2O��
��6��AΪH��CΪN��GΪCl����A��C��G�γɵ����ӻ�����Ļ�ѧʽ��NH4Cl��
�ʴ�Ϊ��NH4Cl��
���� ���⿼��ѧ������ԭ�ӽṹ�Ĺ�ϵ���ƶ�Ԫ�أ�Ȼ��Ԫ�ؼ��仯�����֪ʶ����������⣬�ƶ�Ԫ���ǽ������ѵ㣬Ӧѧ�����ü������֤�ķ��������

| A�� | �٢ޢ� | B�� | �ڢܢ� | C�� | �٢ۢ� | D�� | �ۢܢ� |

| A�� | ͼ�ٱ�ʾ���淴ӦCO��g��+H2O��g��?CO2��g��+H2��g���еġ�H����0 | |
| B�� | ͼ����ʯīΪ�缫����Ȼ���ϡ��Һ�����������������������֮��һ��Ϊ1��1 | |
| C�� | ͼ�۱�ʾ25�棬0.1 mol•L-1����ζ�20 mL 0.1 mol•L-1 NaOH��Һ��pH�����������ı仯 | |
| D�� | ͼ�ܱ�ʾ2SO2��g��+O2��g��?2SO3��g����H��0 �����淴Ӧƽ�ⳣ��K���¶ȵı仯 |
| A�� | ����̼���ƹ��� | B�� | ��������ͭ���� | C�� | �������Һ | D�� | �Ȼ�����Һ |
 ����ͼ��ʾװ��ѡȡ��ͬ�Լ������ʵ�飬����ʵ������Ԥ�ںͽ��۾���ȷ����
����ͼ��ʾװ��ѡȡ��ͬ�Լ������ʵ�飬����ʵ������Ԥ�ںͽ��۾���ȷ����| a | b | ʵ������Ԥ�� | ���� | |
| A | ͭ˿ | Ũ���� | �Թ�c���д�������ɫ���� | ������ǿ������ |
| B | ľ�� | 18.4mol/L���� | ľ����� | Ũ���������Լ������� |
| C | ���� | ����NaCl��Һ | �����з������� | ��������������ʴ |
| D | ��˿ | ������HCl��H2O2��Һ | �Թ�c���д�����ɫ���� | �÷�Ӧ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ˮ�� | B�� | ǿ������ | C�� | ���� | D�� | ��ˮ�� |
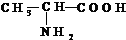 ����ش��������⣮
����ش��������⣮ ��
�� ��CH2=CHCN��CH2=CHCH=CH2��CH2=CHC6H5��
��CH2=CHCN��CH2=CHCH=CH2��CH2=CHC6H5��