��Ŀ����
��֪��������HA��HB�ĵ���ƽ�ⳣ��Ka��HA��>Ka��HB���������й�˵����ȷ����
A������Ũ�Ⱦ�Ϊ0��1 mol��L-1��HA��HB��Һ�У�����Һ��pH��СΪ��pH��HA��>pH��HB��
B����0��1mol��L-1��NaA��Һ�и�����Ũ�ȹ�ϵΪ��c��Na+��>c��A-��>c��OH-��>c��H+��
C�������pH��ͬ��HA��HB��Һ���ֱ�����Ũ�ȵ�NaOH��Һ��ǡ����ȫ��Ӧ���ĵ�NaOH��Һ���HA��HB��
D������Ũ�Ⱦ�Ϊ0��1 mol��L-1��NaA��NaB��Һ�У�����Һ��pH��СΪ��pH��NaA��>pH��NaB��
A������Ũ�Ⱦ�Ϊ0��1 mol��L-1��HA��HB��Һ�У�����Һ��pH��СΪ��pH��HA��>pH��HB��
B����0��1mol��L-1��NaA��Һ�и�����Ũ�ȹ�ϵΪ��c��Na+��>c��A-��>c��OH-��>c��H+��
C�������pH��ͬ��HA��HB��Һ���ֱ�����Ũ�ȵ�NaOH��Һ��ǡ����ȫ��Ӧ���ĵ�NaOH��Һ���HA��HB��
D������Ũ�Ⱦ�Ϊ0��1 mol��L-1��NaA��NaB��Һ�У�����Һ��pH��СΪ��pH��NaA��>pH��NaB��
B
����ƽ�ⳣ��Խ����Խǿ����Ӧ������ˮ��̶Ⱦ�С������AD����ȷ��B��ȷ.pH��ͬʱ��HB��Ũ�ȴ������ĵ��������ƶ࣬C����ȷ����ѡB��

��ϰ��ϵ�д�
�����Ŀ
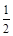 O2 (g) = H2O(l)��H = c kJ/mol
O2 (g) = H2O(l)��H = c kJ/mol H+��SO42��
H+��SO42��