��Ŀ����
3�� ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ�����к��ȵIJⶨ��������0.50mol•L-1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����5.0g��
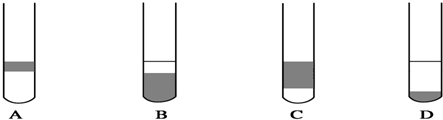
��2������ͼ��ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����abe��
| ���� | ������ƽ �������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��1��д���÷�Ӧ�к��ȵ��Ȼ�ѧ����ʽ�����к���Ϊ57.3kJ•mol-1��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ���������±���
| �¶� ʵ������� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ� ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.6 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.2 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�ڽ�����Ϊ0.50mol•L-1 NaOH��Һ��0.50mol•L-1������Һ���ܶȶ���1g•cm-3���кͺ�������Һ�ı�����c=��4.18��J•��g•�棩-1�����к��ȡ�H=-53.5kJ/mol��ȡС�����һλ����
������ʵ����ֵ�����57.3kJ•mol-1��ƫ�����ƫ���ԭ������ǣ�����ĸ��acd��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��ʵ���и���60mL0.5mol/L�����50mL0.55moi/L�������ƽ��з�Ӧ��������ʵ����ȣ����ų�����������ȣ�����Ȼ���ȣ���ͬ����������к�����ȣ����������к�����ָ�������кͷ�Ӧ����lmolH2O���ų��������������ᡢ��������أ�
���� ��1�����ݹ�ʽm=nM=cVM�������������Ƶ�����������û��245mL������ƿ����250mL������ƿ��
��2����������Ҫ�ڲ��������г��������ݳ������������������õ��������ش�
��1����������кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ��
��2�����ȼ����ÿ����������ⶨ���¶ȲȻ���������ϴ�����ݣ���������¶Ȳ�ƽ��ֵ��
���ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ�
��a��װ�ñ��¡�����Ч�����õ�����ƫС��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ�
�ܷ�Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��� �⣺��1��û��245mL������ƿ��ֻ����250mL������ƿ����Ҫ����NaOH����m=nM=cVM=0.5mol/L��0.25L��40g/mol=5.0g��
�ʴ�Ϊ��5.0��
��2����������Ҫ��С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ף�
�ʴ�Ϊ��abe��
��1����֪ϡǿ�ᡢϡǿ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������ϡ���������������ϡ��Һ����ǿ���ǿ���ϡ��Һ����Ӧ���Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
�ʴ�Ϊ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2����4���¶Ȳ�ֱ�Ϊ��3.5�棬4.0�棬3.9�棬4.1�棬��1���������ϴ����������¶Ȳ�ƽ��ֵ4.0�棻
�ʴ�Ϊ��4.0��
��50mL0.50mol•L-1����������30mL0.50mol•L-1������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ80mL��1g/cm3=80g���¶ȱ仯��ֵΪ��T=4.0�棬������0.025molˮ�ų�������Ϊ��Q=m•c•��T=80g��4.18J/��g•�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-$\frac{1.3376KJ}{0.025mol}$=-53.5kJ/mol��
�ʴ�Ϊ��-53.5kJ/mol��
��a��װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС����a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b����
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ���к��ȵ���ֵƫС����c��ȷ��
d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС����d��ȷ��
�ʴ�Ϊ��acd��
�ܷ�Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.5mol/L�����50mL0.55moi/L�������ƽ��з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�����к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ����Ը���60mL0.5mol/L�����50mL0.55moi/L�������ƽ��з�Ӧ������к�����ֵ��ȣ�
�ʴ�Ϊ������ȣ���ȣ��к�����ָ�������кͷ�Ӧ����lmolH2O���ų��������������ᡢ��������أ�
���� ���⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⣮

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | �ǽ���Ԫ�أ�Si��F��Be | B�� | �Σ����̼李����� | ||
| C�� | ����������ʯ�͡���ˮ������ | D�� | ���ʣ���ǡ����ʯ��ˮ�� |
| A�� | ������̼��ˮ��Ӧ | B�� | Na��ˮ��Ӧ | ||
| C�� | ������ˮ��Ӧ | D�� | ����������ˮ��Ӧ |
| A�� | ��������狀��Ȼ��ƣ�ˮ�� | B�� | �Ȼ������Ȼ�������Һ���۲���ɫ�� | ||
| C�� | ��ˮ��Ӳˮ������ˮ�� | D�� | �ƾ�������ˮ��Ʒ���� |
| A�� | NaAlO2��Al2��SO4��3 | B�� | NaHCO3��Ca��OH��2 | C�� | AlCl3�Ͱ�ˮ | D�� | HCl��Na2CO3 |
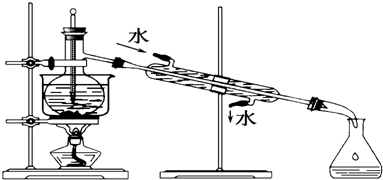
 ��Ȳ�棨H-C=C-CN���е�42.5�棩��һ���Ǽʷ��ӣ�ʵ���ҿ�ͨ����Ȳ���ø����������������ǰ�뿻�������ˮ���Ʊ���
��Ȳ�棨H-C=C-CN���е�42.5�棩��һ���Ǽʷ��ӣ�ʵ���ҿ�ͨ����Ȳ���ø����������������ǰ�뿻�������ˮ���Ʊ���