��Ŀ����
ij��Ȼ��֬A�ķ���ʽΪC57H106O6��1Ħ������֬ˮ��ɵõ�1Ħ�����͡�1Ħ��������֬����B��2Ħ��ֱ������֬����C�����ⶨ��B����Է�������Ϊ280��ԭ�Ӹ�����ΪN��C����N��H����N��O��=9��16��1��
��1��д��B�ķ���ʽ��
��2��д��C�Ľṹ��ʽ��
��3��д����5��̼ԭ�ӵ�C��ͬϵ���ͬ���칹��Ľṹ��ʽ��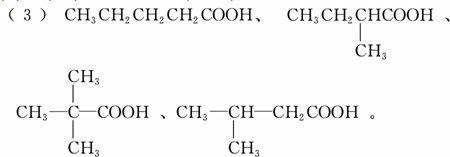
 ��
��
��1��д��B�ķ���ʽ��
C18H32O2
C18H32O2
����2��д��C�Ľṹ��ʽ��
CH3��CH2��16COOH
CH3��CH2��16COOH
��C��������Ӳ֬�ᣨʮ���ᣩ
Ӳ֬�ᣨʮ���ᣩ
����3��д����5��̼ԭ�ӵ�C��ͬϵ���ͬ���칹��Ľṹ��ʽ��


��������1��B�����ʽ��C9H16O����ΪB����Է�������Ϊ280������B�ķ���ʽΪC18H32O2��
��2����֬�Ǹ�֬����ĸ�������1molA��C57H106O6��Ӧ��3mol H2O����ˮ�ⷴӦ������1mol���ͣ�C3H8O3����1molB��C18H32O2����2molC������ԭ�Ӹ����غ�ɵ�C�ķ���ʽΪC18H36O2��C��ֱ������֬���ᣬ��CΪӲ֬�
��3������̼���칹��λ���칹������ͬ���칹�壮
��2����֬�Ǹ�֬����ĸ�������1molA��C57H106O6��Ӧ��3mol H2O����ˮ�ⷴӦ������1mol���ͣ�C3H8O3����1molB��C18H32O2����2molC������ԭ�Ӹ����غ�ɵ�C�ķ���ʽΪC18H36O2��C��ֱ������֬���ᣬ��CΪӲ֬�
��3������̼���칹��λ���칹������ͬ���칹�壮
����⣺��1��B�����ʽ��C9H16O����ΪB����Է�������Ϊ280������B�ķ���ʽΪC18H32O2��
�ʴ�Ϊ��C18H32O2��
��2����Ȼ��֬A��ˮ����Ա�ʾ�ɣ�C57H106O6+3H2O��C3H8O3�����ͣ�+C18H32O2+2C������ԭ���غ�֪��C�ķ���ʽΪ��C18H36O2�����C��ֱ������֬���ᣬ��֪C�Ľṹ��ʽΪ��CH3-��CH2��16-COOH��
�ʴ�Ϊ��CH3-��CH2��16-COOH��
��3����5��̼ԭ�ӵ�Ӳ֬���ͬϵ��Ϊ���ᣨC4H9COOH����������ͬ���칹����4�֣����Ӧ������ҲӦ��4�֣��� ��
��
�ʴ�Ϊ�� ��
��
�ʴ�Ϊ��C18H32O2��
��2����Ȼ��֬A��ˮ����Ա�ʾ�ɣ�C57H106O6+3H2O��C3H8O3�����ͣ�+C18H32O2+2C������ԭ���غ�֪��C�ķ���ʽΪ��C18H36O2�����C��ֱ������֬���ᣬ��֪C�Ľṹ��ʽΪ��CH3-��CH2��16-COOH��
�ʴ�Ϊ��CH3-��CH2��16-COOH��
��3����5��̼ԭ�ӵ�Ӳ֬���ͬϵ��Ϊ���ᣨC4H9COOH����������ͬ���칹����4�֣����Ӧ������ҲӦ��4�֣���
 ��
���ʴ�Ϊ��
 ��
��������������Ҫ�������л������ʽ���ṹ��ʽ��ȷ�����ѶȲ���ע�������غ�����ã�

��ϰ��ϵ�д�
�����Ŀ
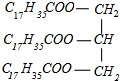 +3NaOH��C17H35COONa+
+3NaOH��C17H35COONa+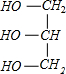
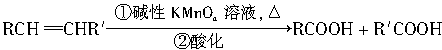 �������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��
�������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol������֬����B��?1molH2��Ӧ�����õ�D��E�Ļ���D��E��Ϊͬ���칹�塣��D��E�Ļ���������KMnO4��Һ�����ữ�õ��������ֲ��