��Ŀ����
��20����90���������������������о�������չʮ��Ѹ�٣���һ�������ڸ߿Ƽ���������ʮ�ֹ�����Ӧ��ǰ���������DZ���һϵ�г����������Ľṹ��ʽ��


�Իش��������⣺
��1��д��������ڡ��ݵķ���ʽ��
��C6H6;��___________;��___________;��___________;��___________��
��2�����黯�����ͨʽΪ___________��
��3��ij������ȤС�飬����ϵ��ʵ�飬���ǽ���ϵ�е�ǰ5�ַ�������һ�ӵ�Ũ��KMnO4������Һ�м��ȡ�������֣�ֻ�б������Ա仯������4�����ʶ���ʹKMnO4������Һ��ɫ��������֪����4�ֳ��������������������ǣ�![]() ��
��![]() �����߶��С��ɴ����ܵó��Ľ�����________________________________��
�����߶��С��ɴ����ܵó��Ľ�����________________________________��
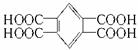
��4���ס��ҡ�����λͬѧͬ��KMnO4������Һ�������屽��ʵ�顣��������Ļ��Һ�ữ����õ�һ����ɫ����X����ȡ0.2540 g X������ˮ���100 mL��Һ�����к͵ζ���ȡ��25.00 mL����Һ����0.1000 mol��L-1 NaOH��Һ�ζ�����ȥ10.00 mLʱ�ﵽ�յ㣻����KMnO4������Һ�������屽����������Ļ��Һ�ữ����õ���ɫ����Y���кͲⶨ֪Y���к�����ֻ��X���к�������һ�룻����KMnO4������Һ�������屽����������Ļ��Һ�ữ�������ɫ���壬���������а����˼�������õ����ֳɷ֣�ͨ����������ȷ��X�Ľṹ��ʽ��
��1��C10H8 C14H10 C18H12 C22H14
��2��C4n+2H2n+4
(3)�����ܱ�KMnO4����
��4��
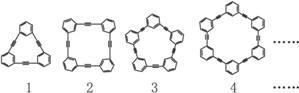
������(1)��ϵ�л�����ÿ����һ��������������о�����һ��C4H2����֪���ķ���ʽ��C6H6���������졢���ı������屽�ķ���ʽ�����ǣ���C10H8����C14H10����C18H12����C22H14��
��2�����ǿ������·������ɳ�ͨʽ��
![]() �ֽ�ɣ�C2H4+C4��1H2��1=C6H6
�ֽ�ɣ�C2H4+C4��1H2��1=C6H6
![]() �ֽ�ɣ�C2H4+C4��2H2��2=C10H8
�ֽ�ɣ�C2H4+C4��2H2��2=C10H8
 �ֽ�ɣ�C2H4+C4��3H2��3=C14H10
�ֽ�ɣ�C2H4+C4��3H2��3=C14H10
 �ֽ�ɣ�C2H4+C4��4H2��4=C18H12
�ֽ�ɣ�C2H4+C4��4H2��4=C18H12
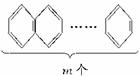 �ֽ�ɣ�C2H4+C4��mH2��m=C4m+2H2m+4
�ֽ�ɣ�C2H4+C4��mH2��m=C4m+2H2m+4
�ʿɹ��ɳ�ͨʽΪC4m+2H2m+4��
��3�����Ļ�ѧ���ʺ��ȶ������ܱ�KMnO4������Һ������
��4����X����Է�������ΪMr�����ӽṹ�к�n���Ȼ������У�
![]()
��֮�ã�Mr=63.5n���������Ϣ֪�������졢���ı������屽����������Ϊ![]() ��
�� �����߶��У���Y���к�������X��һ�룬�����Ƴ�YΪ
�����߶��У���Y���к�������X��һ�룬�����Ƴ�YΪ![]() ,XΪ
,XΪ![]() ��
��

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� �����Լ�дΪ
�����Լ�дΪ ��Ϊ����ԭ�ϣ�������Ӧ�õ�һϵ�еķ�Ȳ��������ṹΪ��
��Ϊ����ԭ�ϣ�������Ӧ�õ�һϵ�еķ�Ȳ��������ṹΪ��

 ����Br2Ϊ��Ҫ��ʼ���ʣ�ͨ���ӳɡ���ȥ������Ӧ�Ƶã�д���ɱ���ϩ��ȡ����Ȳ��������ѧ����ʽ�������Լ���ѡ����
����Br2Ϊ��Ҫ��ʼ���ʣ�ͨ���ӳɡ���ȥ������Ӧ�Ƶã�д���ɱ���ϩ��ȡ����Ȳ��������ѧ����ʽ�������Լ���ѡ����



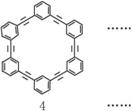
 �����Լ�дΪ�ԣ�
�����Լ�дΪ�ԣ�