��Ŀ����
��14�֣���������������������̥���ϳɶ�����һ�ֵ���A�ķ���ʽΪC4H8��A�⻯����H2�ӳɣ���õ�2�������顣���������գ�
��1��A�Ľṹ��ʽΪ
��2��A���Ծۺϣ�д��A�����־ۺϷ�ʽ���Է�Ӧ����ʽ��ʾ��
��3����Aͨ��������Ȼ�̼��Һ������� ��ԭ��
���÷���ʽ��ʾ��
��4��A��ij�鷢���������Ӧ���ɷ���ʽΪC8H18������B��B��һ±����ֻ��4�֣�
��̼�����Գơ�д��B�Ľṹ��ʽ��
��5����һ����������NBS���ã��£�ϩ������˫��̼����̼ԭ���ϵ�һ����ԭ�ӿɱ���ԭ��ȡ���������ʽΪC4H8��ϩ����һ����������NBS���ã��£��õ���һ���ϩ���� ��
��1��A�Ľṹ��ʽΪ
��2��A���Ծۺϣ�д��A�����־ۺϷ�ʽ���Է�Ӧ����ʽ��ʾ��
��3����Aͨ��������Ȼ�̼��Һ������� ��ԭ��
���÷���ʽ��ʾ��
��4��A��ij�鷢���������Ӧ���ɷ���ʽΪC8H18������B��B��һ±����ֻ��4�֣�
��̼�����Գơ�д��B�Ľṹ��ʽ��
��5����һ����������NBS���ã��£�ϩ������˫��̼����̼ԭ���ϵ�һ����ԭ�ӿɱ���ԭ��ȡ���������ʽΪC4H8��ϩ����һ����������NBS���ã��£��õ���һ���ϩ���� ��
��1��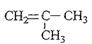
��2��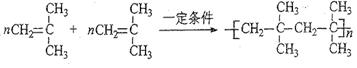

��3���Ⱥ�ɫ��ȥ��CH2=C(CH3)2 + Br2��BrCH2CBr(CH3)2
��4��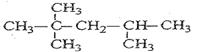
��5��3 ��ÿ��2�֣���14�֣�
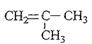
��2��
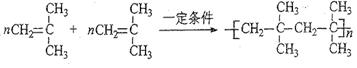

��3���Ⱥ�ɫ��ȥ��CH2=C(CH3)2 + Br2��BrCH2CBr(CH3)2
��4��
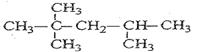
��5��3 ��ÿ��2�֣���14�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��Br2�����ӳɷ�Ӧ�����ɵIJ��ﲻ������(����)
��Br2�����ӳɷ�Ӧ�����ɵIJ��ﲻ������(����)


 ���� ��
���� ��